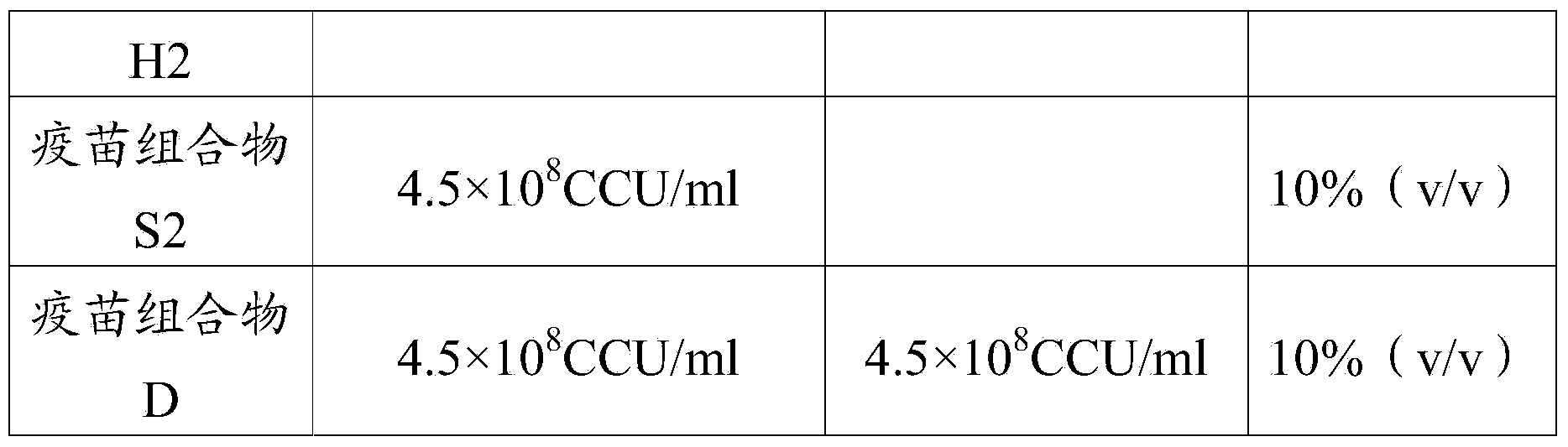Mycoplasma hyorhinis strain, vaccine composition, preparation method and application thereof
A technology of Mycoplasma hyorrhea and vaccine composition, which is applied in the field of veterinary vaccines, can solve the problems of difficulty in screening vaccine strains, differences in the pathogenicity of strains, etc., and achieve the effect of improving the prevention and control effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, the preparation of Mycoplasma hyorhina vaccine composition
[0052] 1. The source of bacteria (virus) strains
[0053] Mycoplasma belongs to Mycoplasma hyorhina LYH strain, which was isolated by our laboratory and preserved in the China Center for Type Culture Collection (CCTCC), address: Wuhan University, Wuhan, China, the preservation number is CCTCC NO: V201334, and the preservation date is August 20, 2013.
[0054] 2. Preparation and inspection of semi-finished products of Mycoplasma hyorhina vaccine
[0055] 2.1 Preparation of seeds for production
[0056] Inoculate the liquid culture medium with 10% freeze-dried strain of Mycoplasma hyorhinis LYH strain, culture at 37°C for 1-3 days, and harvest the bacterial liquid as first-class seeds when the color of the culture medium turns yellow and the pH value drops to 6.8-7.0.
[0057] Inoculate the first-grade seeds with 5% liquid medium, and culture at 37°C for 1-3 days. When the color of the medium tur...
Embodiment 2
[0080] The pathogenicity test of embodiment 2 Mycoplasma hyorhinois LYH strain infection
[0081] In this experiment, the pathogenesis model of artificially infected Mycoplasma hyorhina LYH strain was established, so as to prove that Mycoplasma hyorhina LYH strain can cause the occurrence of swine enzootic pneumonia (SEP).
[0082] 1 Material:
[0083] Animals: 8 to 9 weeks old, pigs with negative serum antibodies to Mycoplasma hyopneumoniae and Mycoplasma hyorhinosus
[0084] Attacking strain: Mycoplasma hyorhinosum LYH strain
[0085] 2 methods:
[0086]10 piglets aged 60-70 days were selected and randomly divided into 2 groups with 5 pigs in each group. Pigs in group 1 were challenged with LYH strain of Mycoplasma hyorhinois, and pigs in group 2 were not challenged as control. After 28 days of observation, the lungs were dissected, and the SEP pneumonia lesions of the experimental pigs were scored according to the 28-point method of Madec and Kobisch (1982). See Table ...
Embodiment 3
[0096] Efficacy test of the Mycoplasma hyorhina vaccine composition of embodiment 3 different antigen content
[0097] The purpose of this test is to evaluate the efficacy of the Mycoplasma hyorhina vaccine composition with different antigen contents against pathogenic Mycoplasma hyorhinophysis infection.
[0098] 1 material
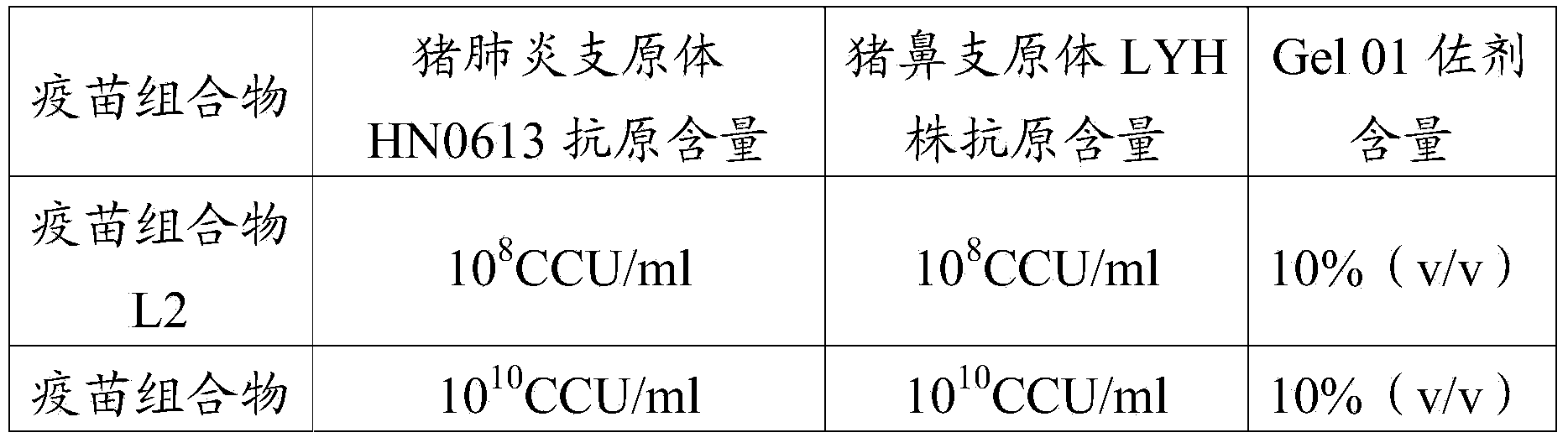
[0099] The vaccine L1 (Mycoplasma hyorhinois LYH strain antigen content of the preparation of embodiment 1 is 10 8 CCU / ml) and vaccine H1 (Mycoplasma hyorhinosus LYH strain antigen content is 10 10 CCU / ml).
[0100] Animals: piglets aged 2 to 3 weeks, with negative serum antibodies to Mycoplasma hyopneumoniae and Mycoplasma hyorhinosus
[0101] Attacking strain: Mycoplasma hyorhinosum LYH strain
[0102] 2 methods:
[0103] Fifteen piglets aged 2-3 weeks were selected and randomly divided into 3 groups with 5 piglets in each group. On the 0th day, inject vaccine L1 into the neck muscle of each pig in the first group, and inject vaccine H1 into the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com