Fusion protein containing mycoplasma hyopneumoniae antigen, vaccine composition and application
A technology of mycoplasma hyopneumoniae and vaccine composition, which is applied to medical preparations containing active ingredients, vaccines, veterinary vaccines, etc., can solve the problems of difficult cultivation of mycoplasma hyopneumoniae, imperfect separation technology, long growth cycle, etc., and achieve Convenient for large-scale production, excellent protective effect, and good immune protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
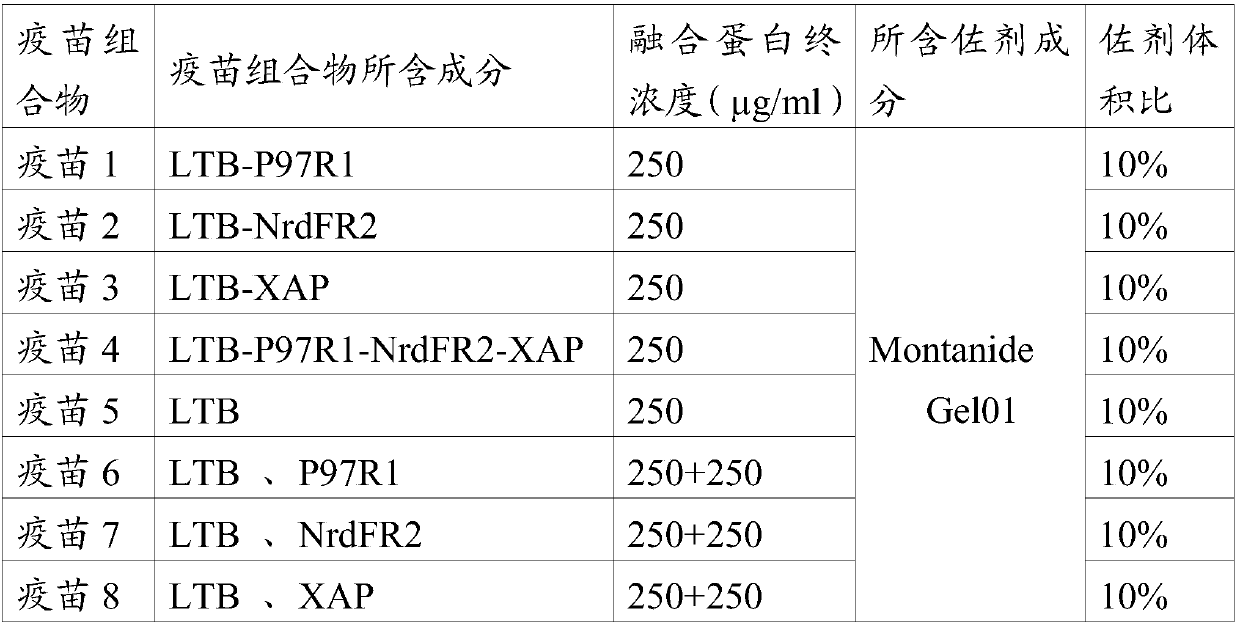
[0037] As an embodiment of the present invention, the content of the fusion protein in the vaccine composition is ≥100 μg / ml.
[0038] As a preferred embodiment of the present invention, the content of the fusion protein in the vaccine composition is 100-400 μg / ml.
[0039]As a more preferred embodiment of the present invention, the content of the fusion protein in the vaccine composition is 250 μg / ml.
[0040] The term "mycoplasma pneumoniae vaccine composition" used in the present invention refers to a vaccine capable of preventing and / or treating diseases or diseases related to infection by Mycoplasma hyopneumoniae. The vaccine that can be used for the vaccine composition of mycoplasma pneumoniae in swine includes (but not limited to) complete or partial mycoplasma pneumoniae cell preparations, inactivated or improved live vaccines, and subunit vaccines.
[0041] The Mycoplasma hyopneumoniae vaccine composition of the present invention is preferably a subunit vaccine, the ...
Embodiment 1
[0082] Example 1, Expression, Identification and Purification of Fusion Protein LTB-P97R1
[0083] 1.1 Construction and identification of LTB-P97R1 fusion gene cloning vector
[0084] According to NCBI ( http: / / www.ncbi.nlm.nih.gov ) gene sequence (see SEQ ID No.1) of enterotoxigenic E. The P97R1 protein gene sequence (see SEQ ID No.3), the primer sequences of LTB protein and P97R1 protein were designed respectively with Primer Premier 5 software, and the upstream primer of LTB protein and the downstream primer of P97R1 protein were respectively added with EcoRI and BamHI digestion site, and the PCR method was used to amplify Escherichia coli heat-labile enterotoxin B subunit (LTB) and Mycoplasma hyopneumoniae P97R1 protein. Details are as follows:
[0085] LTB gene primers were designed as follows:
[0086] Upstream primer: 5'-CGCGAATTCGTCCCCAGACTATTACA-3'
[0087] Downstream primer: 5'-TATACCCATACTGATTG CCGCAAT TGA-3'
[0088] P97R1 gene primers were designed as follow...
Embodiment 2
[0101] Embodiment 2, the preparation of fusion protein LTB-NrdFR2
[0102] According to NCBI ( http: / / www.ncbi.nlm.nih.gov ) gene sequence (see SEQ ID No.1) of enterotoxigenic E. The NrdF protein gene sequence (see SEQ ID No.5), use Primer Premier 5 software to design the primer sequences of LTB protein and NrdF protein respectively, and add EcoRI, HindIII enzyme digestion respectively at the upstream primer of LTB protein and the downstream primer of NrdFR2 protein site, and the PCR method was used to amplify Escherichia coli heat-labile enterotoxin B subunit (LTB) and Mycoplasma hyopneumoniae NrdFR2 protein. Details are as follows:
[0103]LTB gene primers were designed as follows:
[0104] Upstream primer: 5'-CGCGAATTCGTCCCCAGACTATTACA-3'
[0105] Downstream primer: 5'-TATACCCATACTGATTG CCGCAAT TGA-3'
[0106] NrdFR2 gene primers were designed as follows:
[0107] Upstream primer: 5'-GATCTATTATATAAACTAATTG-3'
[0108] Downstream primer: 5'-CCCAAGCTTTTAAAACTCCCAATTCTT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com