Mycoplasma pneumoniae fusion antigen as well as preparation method and application thereof
A technology of Mycoplasma pneumoniae and fusion antigen, which is applied in the biological field, can solve the problems of strict preparation process requirements, uneven product quality, human infection, etc., and achieves the effects of broad market prospects, high sensitivity and strong specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Synthesis of target gene encoding P1M / P30A antigen and construction of recombinant vector
[0068] 1. Use software such as ProtScale to analyze the entire amino acid sequence of Mycoplasma pneumoniae (FH strain) antigens P1 and P30, screen out the antigenic protein sequences P1M: residues 1341-1518 and P30A: residues 170-182, and connect them with short peptides.
[0069] Design PCR primers HP-P1M / P30A-P1M-for, the sequence is:
[0070] CATCACAGCAGCGGCTGGCTGGTTGGCCAG,
[0071] and HP-P1M / P30A-P1M-rev, the sequence is:
[0072] CGGAAAACCGGTACGCTGCAGGGTCTGCGGACCTTG, use the recombinant plasmid pET28a-P1F (P1 antigen fragment residues: 1181-1525) preserved in our laboratory as a template to amplify the P1M nucleic acid fragment; the PCR amplification reaction system is:
[0073]
[0074] The PCR amplification reaction conditions are:
[0075] Pre-denaturation: 98°C, 2min;
[0076] Deformation, annealing, elongation: 98°C, 10s; 60°C, 20s; 72°C, 25s; cycle ...
Embodiment 2
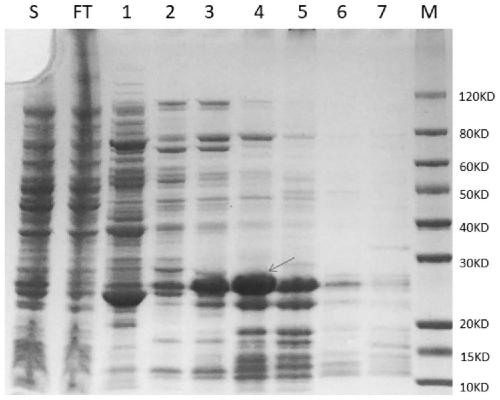
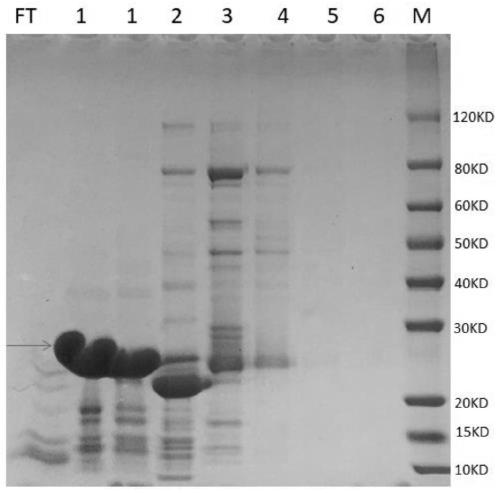
[0105] Example 2: Induced expression and purification of Mycoplasma pneumoniae fusion antigen (P1M / P30A antigen)
[0106] 1, the recombinant vector P1M / p30A-pET28a among the embodiment 1 is transformed into Escherichia coli E.coli Rosetta (DE3) competent cell, in the LB containing the kanamycin of 50 μ g / ml and the chloramphenicol of 50 μ g / ml Cultivate on a culture plate at 37°C for 14-16 hours; screen positive recombinant bacteria, pick a single colony and inoculate it into 5ml LB medium containing 50μg / ml kanamycin and 50μg / ml chloramphenicol, and culture overnight .
[0107] 2. Inoculate the bacterium solution of the above-mentioned overnight culture into the 750ml LB medium containing the kanamycin of 50 μg / ml and the chloramphenicol of 50 μg / ml by 1% (v / v) inoculation amount, 37 ℃, 250rpm, Cultivate to bacterial solution OD 600 1.0-1.3, and then induced with IPTG with a final concentration of 0.5mM-1.0mM at 25°C and 250rpm for 3-4h.
[0108] 3. Collect the 0.75L mediu...
Embodiment 3
[0122] Embodiment 3: the preparation of other several mycoplasma pneumoniae antigens
[0123] Our laboratory also prepared P1M antigen (P1 antigen fragment: residues 1341-1518) and P1C antigen (P1 antigen fragment: residues 1288-1518).
[0124] Our laboratory also prepared several other fusion antigens of Mycoplasma pneumoniae, including P1M / P30C antigen, P1C / P30C antigen and P1C / P30A antigen, using a method similar to that of Examples 1 and 2.
[0125] 1. Preparation of P1M antigen: Use software such as ProtScale to analyze the entire amino acid sequence of Mycoplasma pneumoniae (FH strain) antigen P1, and screen out the protein sequence P1M containing antigenicity: residues 1341-1518. After codon optimization for Escherichia coli, use the whole gene The recombinant plasmid pET28a-P1M expressing P1M antigen was obtained by synthetic method, and transformed into Escherichia coli E. coli Rosetta (DE3); the expression and purification of the target protein P1M were induced.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com