Mycoplasma bovis secretory protein MbovP0145 and application thereof
A technology of mycoplasma bovis and protein, which is applied in the application field of mycoplasma bovis secretory protein MbovP0145, its antibody, and the preparation of mycoplasma bovis detection kit, which can solve the problems of inapplicable clinical batches, rapid and early detection, time-consuming and labor-intensive, and easy pollution And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Cloning and expression of Mycoplasma bovis Mbov_0145 gene
[0021] 1.1 Cloning of Mycoplasma bovis Mbov_0145 gene
[0022] Due to the codon preference of Escherichia coli, the codon UGA encoding tryptophan in Mycoplasma bovis is used as a terminator in Escherichia coli, therefore, when expressing the Mycoplasma bovis gene with Escherichia coli, the mycoplasma gene needs to be mutated so that The codon UGA was mutated to the codon UGG for expression of tryptophan in E. coli.
[0023] The applicant isolated a local strain of Mycoplasma bovis from diseased lung tissue of sick cattle in June 2008, and named it Mycoplasma bovis HB0801 (Mycoplasma bovis HB0801), which has been disclosed in the patent literature of CN 102220263A.
[0024] According to the codon preference of Escherichia coli, the Mbov_0145 gene in Mycoplasma bovis HB0801 (genome GenBank accession number is CP002058) is modified by codons. , 1284, 1359 synonymous mutations of 11 codons occurred,...
Embodiment 2
[0039] Example 2: Identification of the secretion properties of the Mycoplasma bovis protein MbovP0145
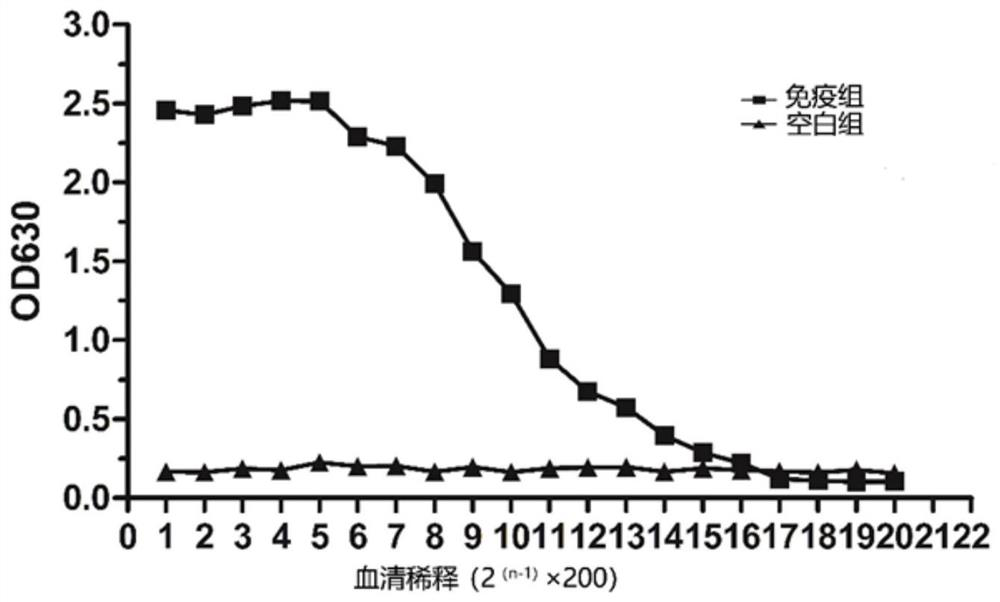
[0040] 2.1 Preparation and titer detection of mouse polyclonal antibody against recombinant Mycoplasma bovis rMbovP0145 protein
[0041] Using the rMbovP0145 protein prepared above to immunize BALB / C mice, the immunization procedure is as follows:
[0042] (1) For the first immunization, the dose of Mycoplasma bovis rMbovP0145 antigen was 100 μg / mouse, and Freund's complete adjuvant was added to subcutaneously inject multiple points on the back of the neck, 0.2 mL / mouse.
[0043] (2) Two weeks later, the second immunization, the dose of Mycoplasma bovis rMbovP0145 antigen was 100 μg / mouse, and Freund's incomplete adjuvant was added to subcutaneously inject multiple points on the back of the neck, 0.2 mL / mouse.
[0044] (3) Four weeks later, for the third immunization, the dose of Mycoplasma bovis rMbovP0145 antigen was 100 μg / mouse, and Freund's incomplete adjuvant was add...
Embodiment 3
[0052] Example 3: Identification of antigenicity of Mycoplasma bovis protein MbovP0145
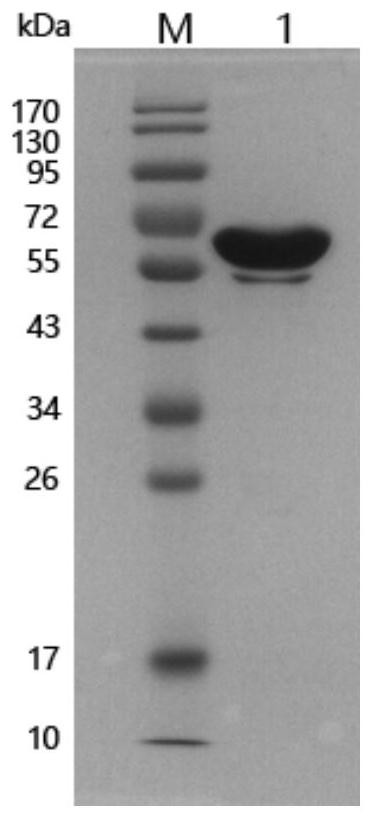
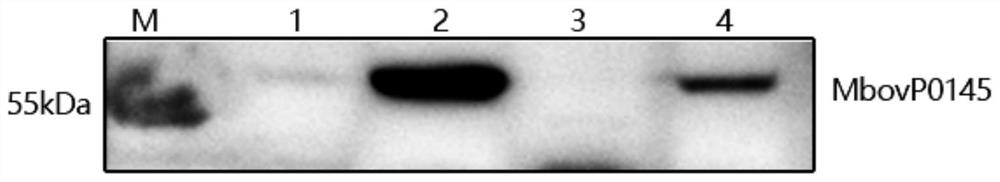
[0053] The antigenicity identification of rMbovP0145 protein was carried out by western blot method. The main steps were as follows: SDS-PAGE electrophoresis was performed on the purified recombinant protein rMbovP0145, and the protein on the gel was transferred to PVDF membrane. Positive serum and bovine negative serum were used as the primary antibody for incubation, and HRP-labeled goat anti-bovine IgG was used as the secondary antibody for western blot verification.
[0054] The results show that MbovP0145 can react with the positive serum of Mycoplasma bovis, and there is a band at the corresponding protein size of 55kDa ( Figure 4 A), consistent with the expected molecular weight of MbovP0145, but no obvious reaction with the negative serum of Mycoplasma bovis ( Figure 4 B).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com