Vaccine freeze-drying protective agent containing no gelatin and human albumin
A freeze-dried protective agent and vaccine technology, which is applied in the direction of freeze-dried transportation, medical preparations containing active ingredients, antiviral agents, etc., can solve problems such as prone to failure, decreased vaccine stability, and unsatisfactory vaccine protection effect. Achieve the effect of improving safety and reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
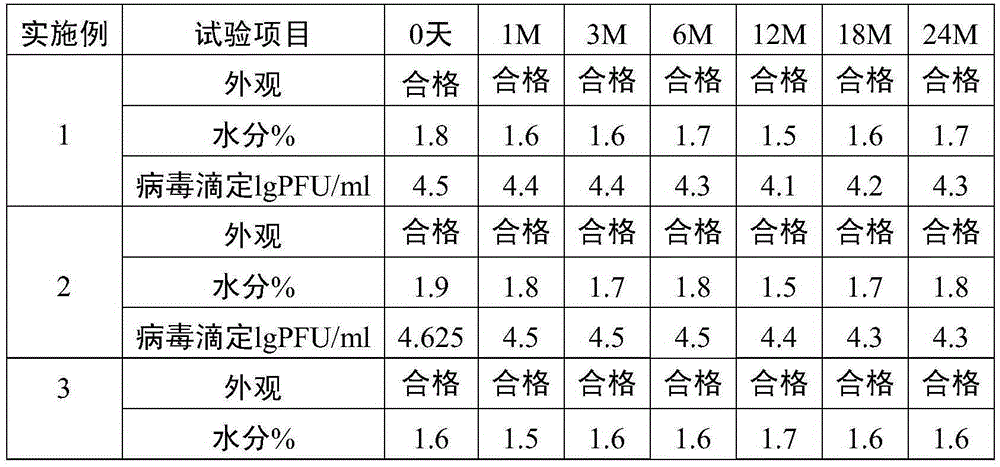
Embodiment 1
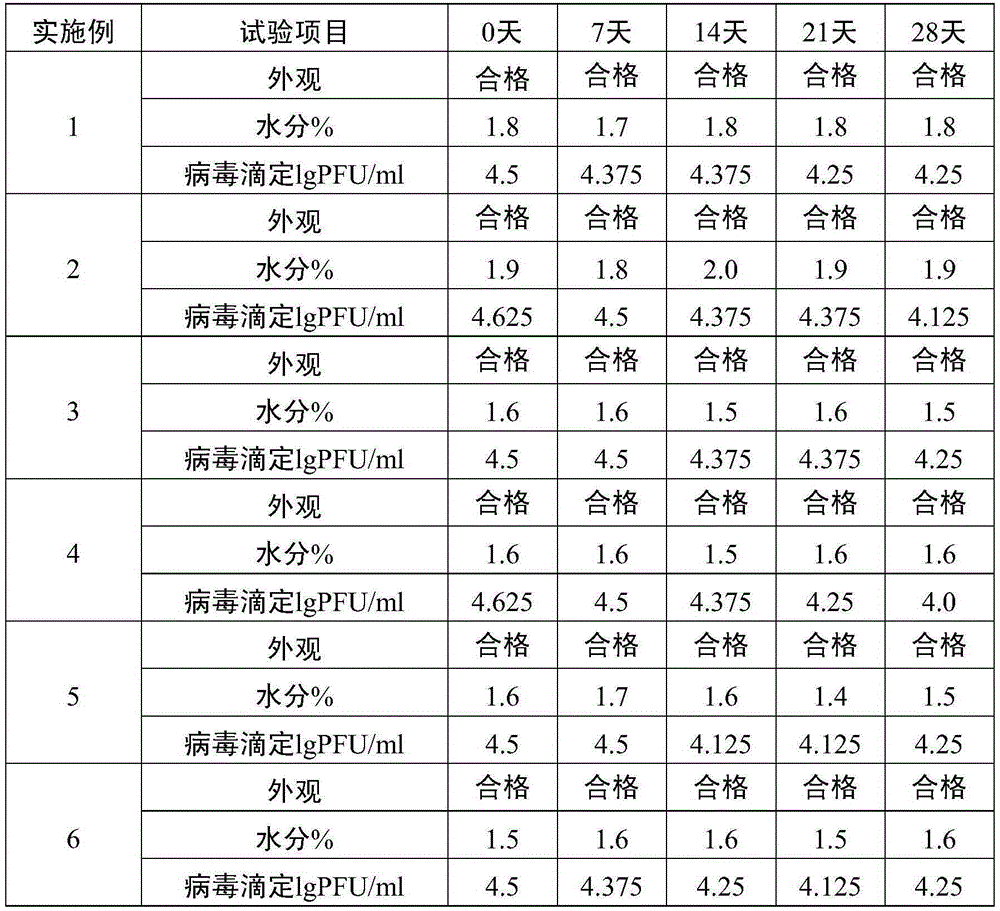
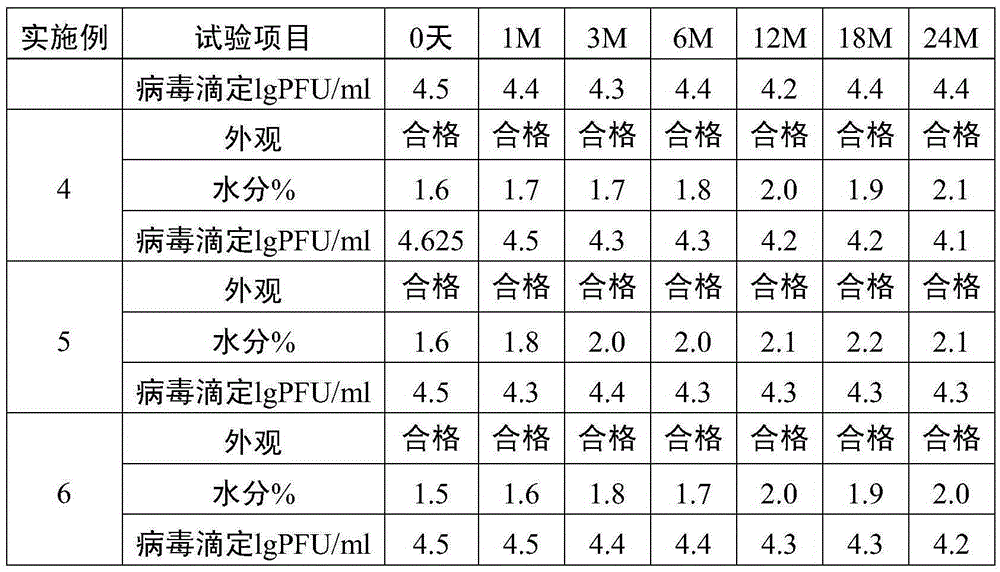
[0031] (1) Take a single harvest liquid of live attenuated varicella vaccine, add a freeze-drying protective agent to remove cell fragments, and obtain a stock solution of live attenuated varicella vaccine. The matrix solution of the protective agent is PBS buffer solution, and the final concentration of the protective agent in the stock solution of the live attenuated varicella vaccine is 1.5% sucrose, 3.5% dextran 70, 2% sorbitol, 0.8% sodium glutamate, 1% L-arginine acid.
[0032] (2) Add appropriate amount of diluent (containing 1.5% sucrose, 3.5% dextran 70, 2% sorbitol, 0.8% sodium glutamate, 1% L -Arginine) formulated into vaccine semi-finished products. The vaccine semi-finished product contains the following final concentration components: 1.5% sucrose, 3.5% dextran 70, 2% sorbitol, 0.8% sodium glutamate, and 1% L-arginine.
[0033] (3) The semi-finished product of the vaccine is divided into vials and freeze-dried to obtain the finished product of live attenuated v...
Embodiment 2
[0035] A single harvest liquid of live attenuated varicella vaccine is taken, and a protective agent is added to remove cell fragments to obtain stock solution of live attenuated varicella vaccine. The matrix solution of the protective agent is water for injection, and the final concentration of the protective agent in the stock solution of the live attenuated varicella vaccine is 5% sucrose, 3% dextran 70, 1% sorbitol, 1% sodium glutamate, 0.2% L-arginine acid.
[0036] Add appropriate amount of diluent (containing 5% sucrose, 3% dextran 70, 1% sorbitol, 1% sodium glutamate, 0.2% L-arginine) to the stock solution of varicella live attenuated vaccine according to the virus titer of 4.8lgPFU / ml Prepared into vaccine semi-finished products. The vaccine semi-finished product contains the following final concentration components: 5% sucrose, 3% dextran 70, 1% sorbitol, 1% sodium glutamate, 0.2% L-arginine.
[0037] The semi-finished product of the vaccine is divided into vials a...
Embodiment 3
[0039] (1) Take a single harvest liquid of live attenuated varicella vaccine, add a protective agent to remove cell fragments, and obtain the stock solution of live attenuated varicella vaccine. The matrix solution of the protective agent is 199 culture solution, and the final concentration of the protective agent in the stock solution of the live attenuated varicella vaccine is 10% sucrose, 1.5% dextran 70, 2% sorbitol, 1.2% sodium glutamate, 0.5% L-alcohol acid. The manufacturer of 199 culture medium is GIBCO.
[0040] (2) Add appropriate amount of diluent (containing 10% sucrose, 1.5% dextran 70, 2% sorbitol, 1.2% sodium glutamate, 0.5% L-alcohol) to the stock solution of varicella live attenuated vaccine according to the virus titer of 4.8lgPFU / ml Amino acid) formulated into vaccine semi-finished products. The vaccine semi-finished product contains the following final concentration components: 10% sucrose, 1.5% dextran 70, 2% sorbitol, 1.2% sodium glutamate, 0.5% L-argin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com