Duck virus hepatitis strains and inactivated vaccine
A technology for duck viral hepatitis and inactivated vaccines, which is applied in antiviral agents, viruses/bacteriophages, biochemical equipment and methods, etc., can solve the problems of breeding losses, local outbreaks of duck viral hepatitis, etc., and achieve good immune effects , normal feeding, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of Duck Viral Hepatitis Virus Strain (DHV YC)
[0038](1) Disease material treatment: Duck farms in Henan where duck viral hepatitis occurred, collected duck liver disease materials with obvious lesions, washed 3 times with sterilized PBS containing 100IU / ml penicillin and 100mg / ml streptomycin double antibodies , Dilute and grind with sterilized PBS 5 times the weight of the disease material. Freeze and thaw the prepared homogenate at -20°C, freeze and thaw twice at 20°C, centrifuge at speeds of 3000r / min and 8000r / min for 15min, and shake the supernatant with chloroform at room temperature for 10-15min. The supernatant was collected by centrifugation, filtered through a 0.22 μm filter membrane, aliquoted, and stored at -70°C.
[0039] (2) Establishment of chicken embryo-adapted strain of duck hepatitis virus: the virus suspension obtained in step (1) is diluted 1:100 with sterilized PBS, and 20 pieces of 9-day-old SPF chicken embryos are inoc...
Embodiment 2
[0040] Example 2 Preparation of Inactivated Vaccine of Duck Viral Hepatitis
[0041] (1) Dilute the DHV YC strain 100 times with sterilized PBS, inoculate it into 9-day-old SPF chicken embryos, and collect the chicken embryo fluid that died within 48-96 hours and had obvious lesions.
[0042] (2) Inactivation: add 0.05% (mass percentage) of β-propiolactone to the qualified chicken embryo liquid, inactivate at 4°C for 24h, and then hydrolyze it for 2h at 37°C in a water bath, as an antigen for making seedlings liquid.
[0043] (3) Preparation of vaccine by emulsification: oil phase preparation: take 94ml of white oil, 6ml of Siben-80, mix well and add 1.5g of aluminum stearate. Preparation of aqueous phase: Take 20ml of antigen solution, 3.6ml of Tween-80, 1.5g of immune enhancer isoprinosine, 4.9ml of sterilized saline, and mix well. Take 100ml of the oil phase and place it in an emulsification tank, pre-emulsify at 7500r / min for 3min, and slowly add 50ml of water phase a...
Embodiment 3
[0045] Example 3 Test of Duck Viral Hepatitis Vaccine
[0046] (1) Physical properties
[0047] Appearance milky white emulsion
[0048] Dosage Form Water-in-Oil
[0049] Viscosity Use a 1ml straw (outlet diameter: 1.2mm) to suck up the vaccine at about 25°C, let it flow out vertically and naturally, record the time required for the outflow of 0.4ml, which is 4.8s, which meets the regulations.
[0050] Stability Store at 37°C for 21 days or put 5ml of the vaccine into a centrifuge tube and centrifuge at 3000r / min for 15min without breaking the emulsion.
[0051] (2) Sterility test was carried out according to the appendix of the current "Chinese Veterinary Pharmacopoeia", and there was no bacterial growth.
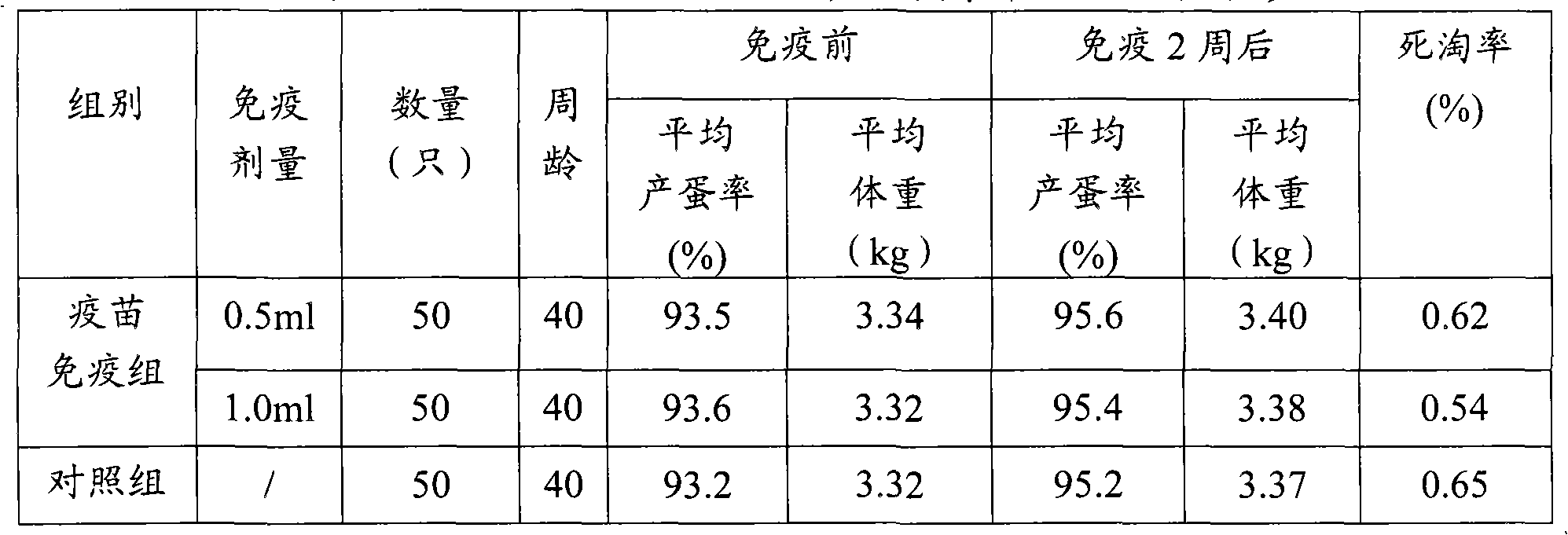
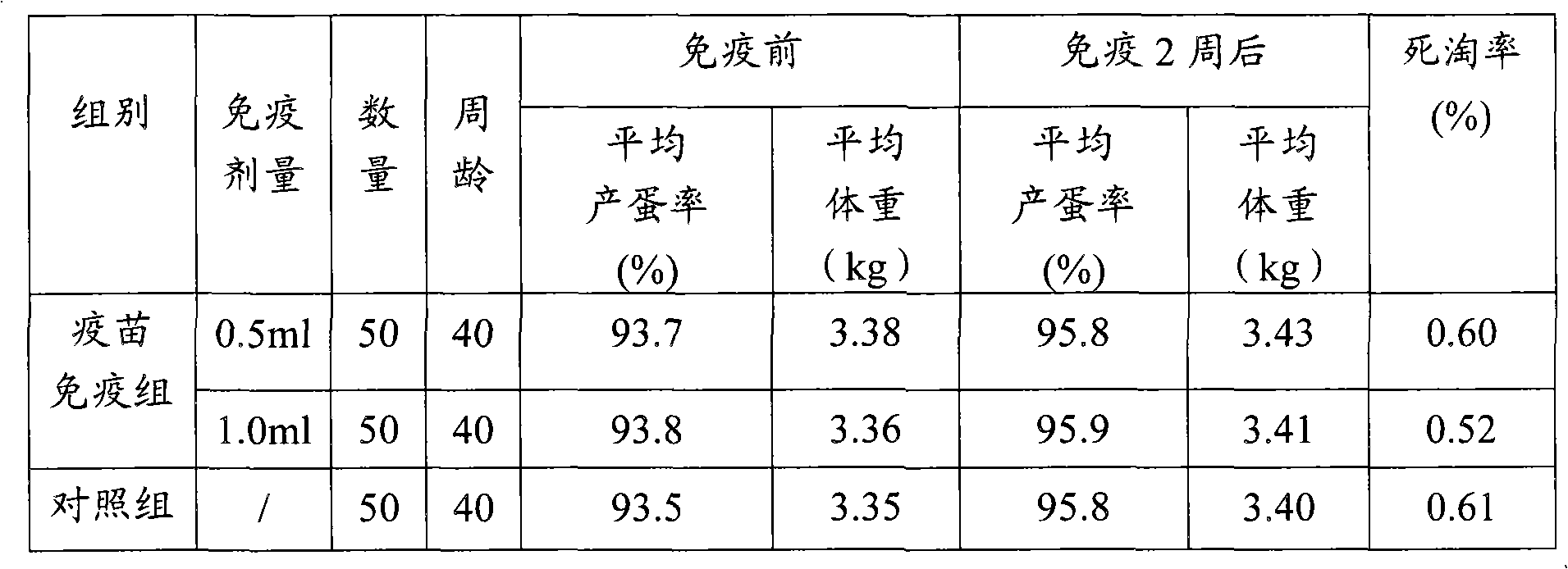
[0052] (3) Safety inspection Take 10 1-day-old Cherry Valley ducks, inject 1ml of vaccine subcutaneously in each neck, observe for 14 days, no local and systemic adverse reactions caused by vaccine injection appear.
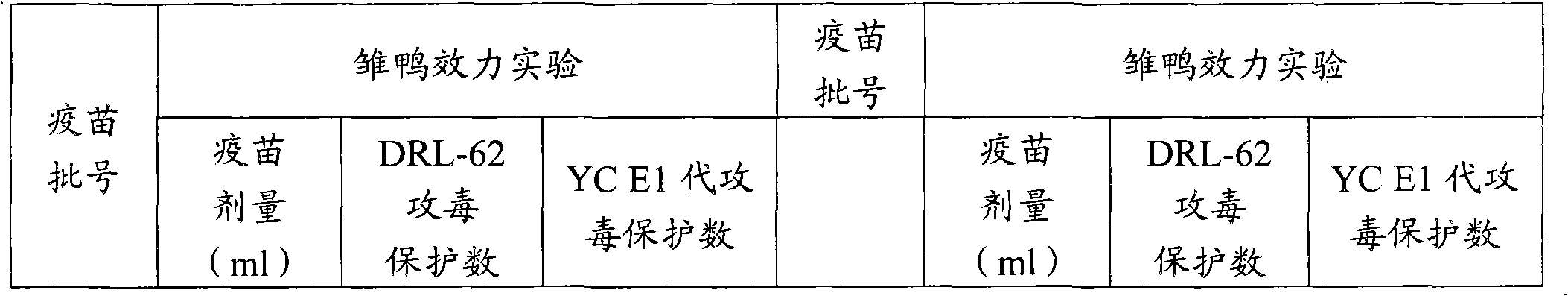
[0053] (4) Efficacy test 10 1-day-old Cherry Val...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com