Porcine epidemic diarrhea vaccine strain and vaccine containing it
A porcine epidemic diarrhea and vaccine virus technology, applied in the field of virus strains and vaccines containing the virus strain, can solve the problems of difficult to meet the requirements of neutralizing antibody levels, poor stability of vaccine virus strains, and insufficient immunogenicity, so as to prevent porcine Epidemic diarrhea infection, good immunogenicity, good passive protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 porcine epidemic diarrhea vaccine strain
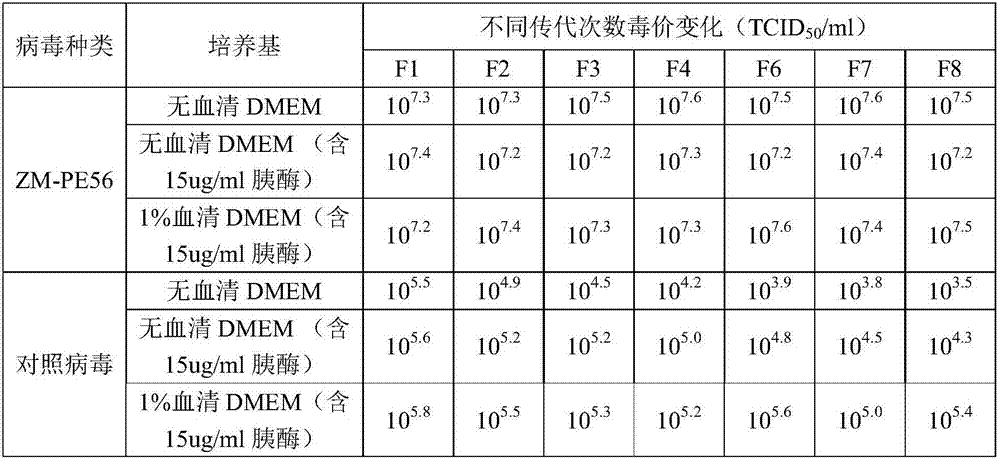
[0023] (1), Cloning Screening of Porcine Epidemic Diarrhea Vaccine Strain ZM-PE56
[0024] Porcine epidemic diarrhea vaccine strain ZM-PE56 was obtained by 5 plaque clones and 6 passages. Its cloning method is as follows:
[0025] Form a monolayer of Vero cells and plate a 6-well plate.
[0026] Take 100 μL of PEDV virus liquid, dilute it with diluent according to 10-fold serial dilution, and dilute 10 -6 、10 -5 、10 -4 、10 -3 、10 -2 The diluted virus solution was inoculated in a 6-well plate covered with a monolayer of Vero cells, each well was connected with 0.5ml, and a negative control well without the virus was set at the same time.
[0027] Discard the cell culture medium in the cell culture plate before inoculation, rinse the six-well plate twice with plate washing solution, and then discard it.
[0028] After adsorption at 37°C for 1.5h (shake once every 30 minutes), the virus liqu...
Embodiment 2
[0042] The preparation of embodiment 2 porcine epidemic diarrhea vaccine
[0043](1) Antigen preparation
[0044] PEDV production seeds (virus content ≥ 10 6.0 TCID 50 / ml), according to 0.1% ~ 1% of the culture volume inoculated to form a single layer of Vero cells, add DMEM maintenance solution for culture, when 75% of the cells have lesions (about 72h), freeze and thaw twice to harvest the virus liquid, centrifuge Or the supernatant collected after filtration is the antigen. Harvested antigens were serially diluted 10-fold and inoculated into 96-well plate Vero cell monolayers, each dilution was inoculated into 8 wells, observed for 4 days, and the virus content was calculated according to the Reed-Muench method formula. After determination, the content of one batch of antigenic virus cultivated was 10 7.5 TCID 50 / ml.
[0045] (2) The preparation of the finished vaccine product uses pure water as a solvent, and prepares hydrolyzed gelatin containing 3%, 1% casein hyd...
Embodiment 3
[0048] Embodiment 3 stability test
[0049] This embodiment is used to illustrate the stability of the prepared vaccine of the present invention, and specific operations are as follows:
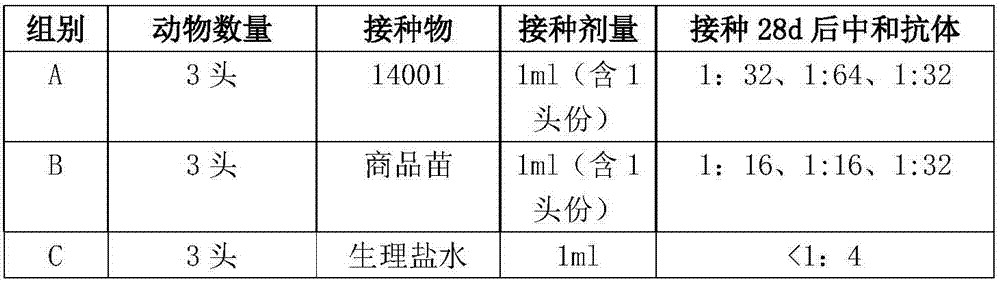
[0050] The two batches of vaccines 14001 and 14002-CG prepared in Example 2 were placed at 37°C for 7 days to measure the change in toxicity, and the results showed that the toxicity of the former decreased by 10 0.7 TCID 50 , while the latter drops by 10 1.5 TCID 50 , indicating that the addition of suitable concentrations of hydrolyzed casein, arginine hydrochloride, sodium glutamate and other ingredients in the new protective agent can significantly improve the stability of the vaccine.
[0051] The two batches of vaccines 14001 and 14002-CG prepared in Example 2 were placed in a refrigerator at -15°C, and the changes in toxicity were measured at 0, 12, 24 and 36 months respectively. As a result, at different time points, the virus content of each vaccine in 14001 batches of vaccines w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com