Infectious clone containing rabies virus glycoprotein and application
A technology for infectious cloning and rabies virus, which is applied to infectious cloning and application fields containing rabies virus glycoprotein, can solve problems such as unclear mechanism, and achieve the effects of rapid proliferation, high success rate and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Obtaining of an infectious clone containing rabies virus glycoprotein:
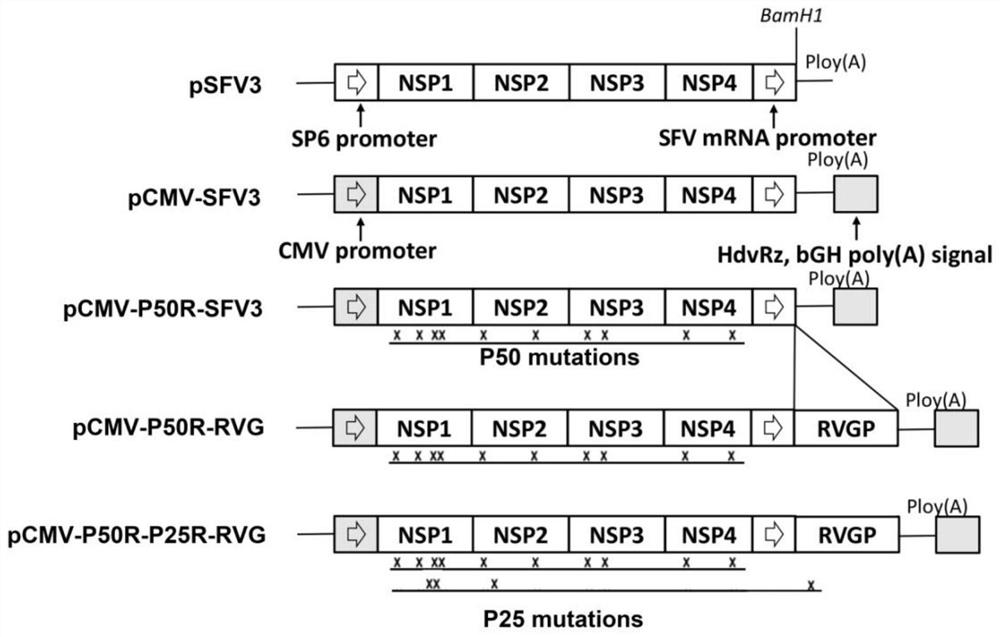
[0030] 1) Construction of Semliki forest virus replicon driven by CMV promoter: pSF V3 (purchased from addgene) was transformed by using multi-segment homologous recombination technology, the original SP6 promoter was replaced by CMV promoter, and multimer Insert the hepatitis D virus ribozyme sequence (HdvRz) and bovine auxin polyadenylation signal sequence (bGH poly(A) signal) behind the adenine tail, eliminating the step of in vitro transcription, the specific method is as follows:
[0031] 4 pairs of homologous recombination primers were designed (TY-A-F / R, TY-B-F / R, TY-C-F / R, TY-D-F / R in Table 1), wherein TY-A-F / R is to amplify HdvRz and bGH The primers for poly(A) signal, TY-C-F / R are the primers for amplifying CMV promoter (the template plasmid BAC containing CMV promoter, HdvRz and bGH poly(A) signal was kindly provided by Professor Peng Guiqing of Huazhong Agricultural University, Wang G ...
Embodiment 2
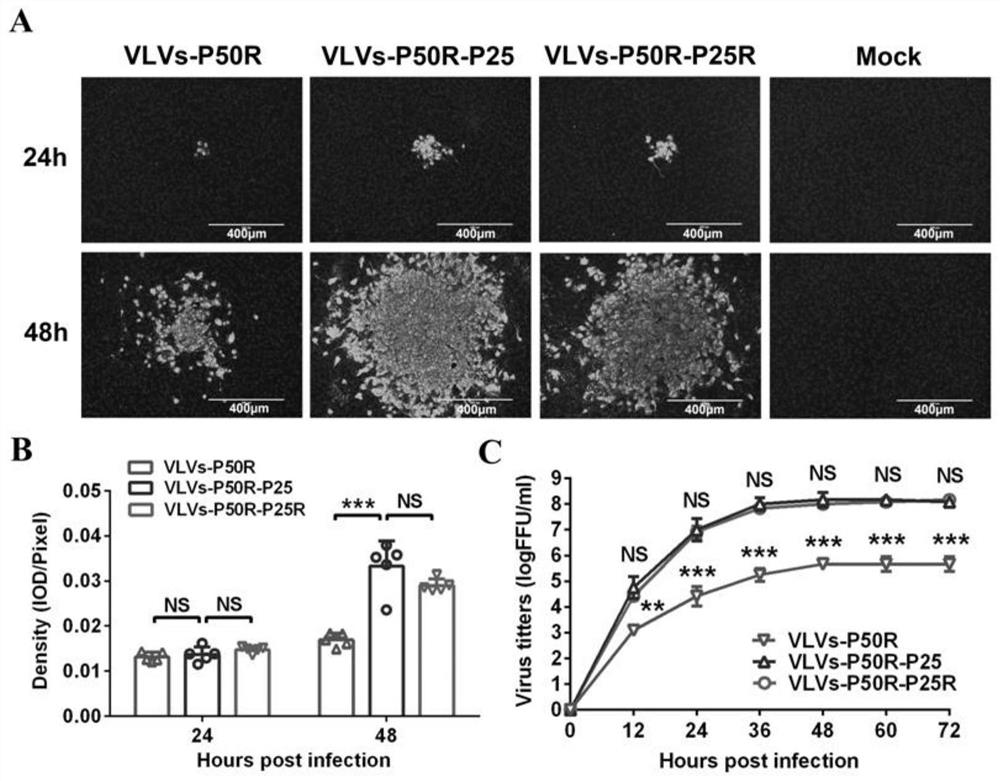
[0053] The rabies virus-like vesicles VLVs-P50R, VLVs-P50R-P25 and VLVs-P50R-P25R virus fluorescent spot diffusion and growth curve determination provided by the invention:
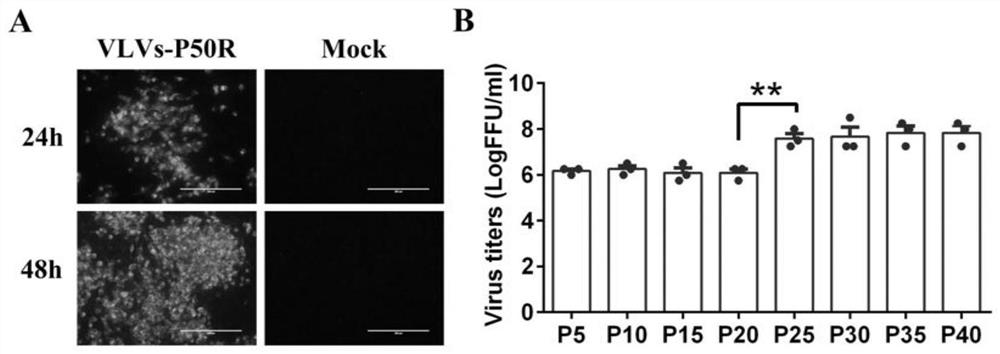
[0054] Fluorescent spot diffusion assay of virus-like vesicles: mix 1 x 10 5 BHK-21 cells were inoculated into each well of a 24-well plate, and when the confluence of the cells reached 90%, VLVs-P50R, VLVs-P50R-P25 or VLVs-P50R-P25R were inoculated at MOI=0.001, 37°C, 5% CO 2 After adsorbing in the incubator for 1 h, wash with PBS three times, add 2% FBS-containing DMEM medium and 2% methylcellulose cover and store at 37°C, 5% CO 2 Cultured in an incubator, took out a plate at 24h and 48h respectively, discarded the cover, washed three times with PBS, fixed with pre-cooled 80% acetone at -20°C for 20min, and then observed the formed cells by indirect immunofluorescence. Fluorescent spots ( image 3 Middle A). In the experimental group, 5 fluorescent spots were randomly selected in each well, and the p...
Embodiment 3
[0057] Purification, SDS-PAGE and Western Blot of rabies virus-like vesicles VLVs-P50R, VLVs-P50R-P25, VLVs-P50R-P25R provided by the invention:
[0058] Purification and SDS-PAGE of VLVs-P50R, VLVs-P50R-P25 and VLVs-P50R-P25R: Ultracentrifuge the collected VLVs-P50R, VLVs-P50R-P25 and VLVs-P50R-P25R at 30,000rpm at 4°C for 2.5 h (Beckman, SW32Ti), discard the supernatant, add 1ml PBS to the bottom of the tube, let it dissolve overnight at 4°C, take 100μl, add 5×SDS loading buffer and boil it for SDS-PAGE detection, and at the same time use purified LBNSE The virus is used as a marker, and the rest of the concentrated virus is subjected to 20%-60% sucrose density gradient centrifugation, and a precipitate layer appears at a sucrose concentration of 40%. Carefully absorb this layer of precipitate, add PBS to dilute, and perform ultra-high-speed centrifugation again to remove sucrose, and finally use 100ul PBS was dissolved at 4°C overnight, and frozen at -80°C for later use. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com