Neutralizing epitope of human adenovirus type 3 (HAdV-3) and type 7 (HAdV-7) and application thereof
An antigenic epitope, adenovirus technology, applied in the direction of application, antiviral agent, antiviral immunoglobulin, etc., can solve the problem of inability to prevent other types of adenovirus infection, adenovirus vaccine strain infection, and inability to effectively resist 3 adenovirus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
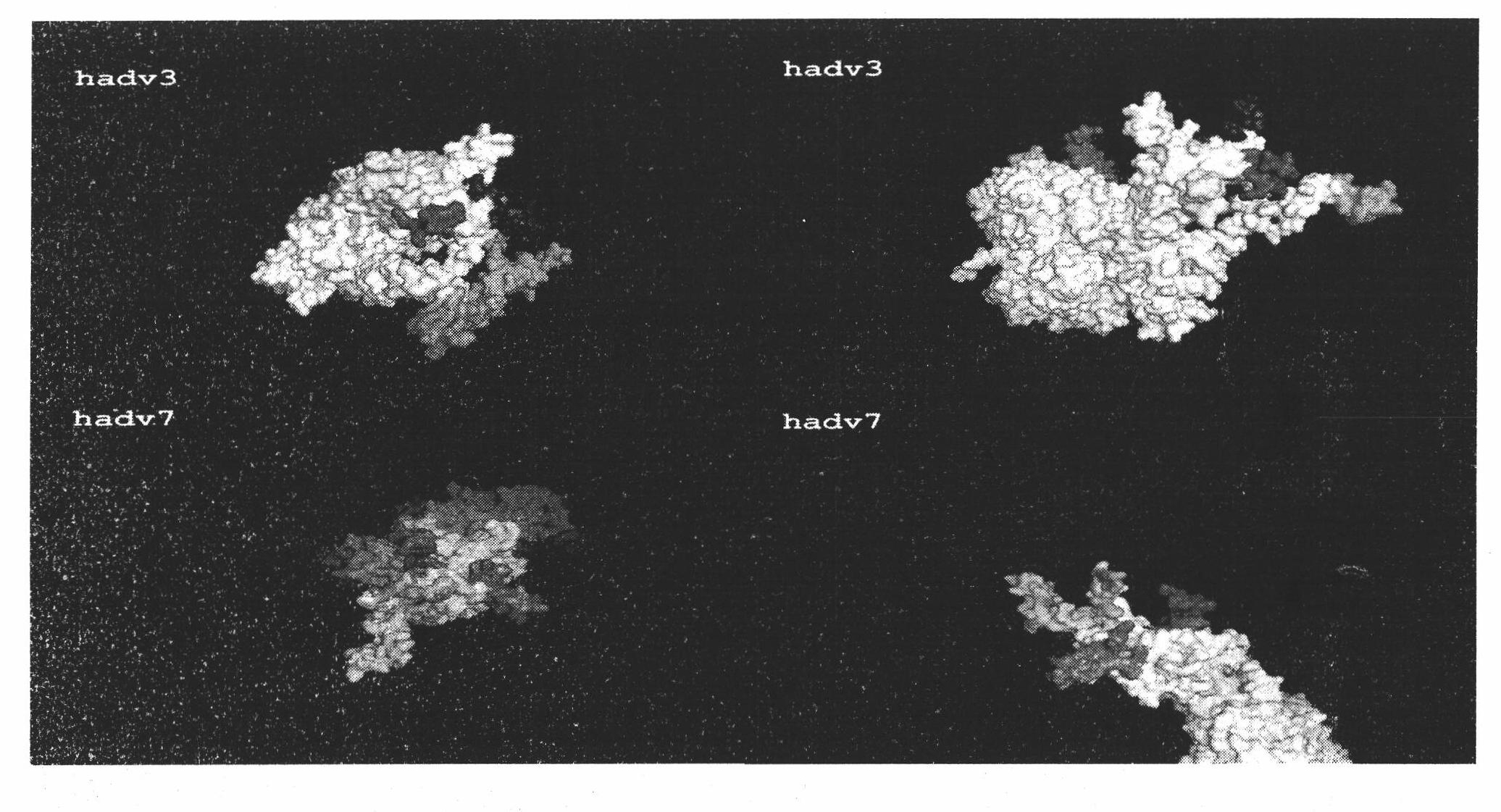
[0020] Example 1: Bioinformatics analysis predicts hypervariable regions (HypervariableRegions, HVRs) of human adenovirus
[0021] The specific steps of the bioinformatics analysis are as follows: firstly, the amino acid sequence of the human adenovirus hexon protein was compared, and then the possible neutralizing antigen epitope was predicted by using the "Antigenic" tool in Emboss. Then, the 3D three-dimensional structure of the human adenovirus type 3, 7 hexon was modeled and the potential neutralizing epitope on the 3D structure was mapped. Finally, the sites exposed on the surface of the hexon "loop" were found and localized to non-constant regions (hypervariable regions) on the sequence.
[0022] figure 1 It is a three-dimensional diagram of the above analysis results, row A is a top view, and row B is a side view. by right figure 1 By analyzing the results, the potential neutralizing epitope on the human adenovirus type 3, 7 hexon can be located and its amino acid s...
Embodiment 2
[0033] Embodiment 2: construct recombinant virus
[0034] In order to analyze the predicted neutralizing epitopes of the hypervariable region (HVR) of type 3 and type 7 human adenoviruses through serum neutralization test and challenge test, the inventors used overlapping PCR (overlapping PCR) method to construct recombinant virus.
[0035] The HVR1, 2, 5 and 7 sequences of type 7 adenovirus were replaced on the PBR322-L-R plasmid by overlapping PCR (overlapping PCR), and then homologously recombined with the PBR-Adv3-EGFP plasmid. The specifically obtained recombinant plasmids are PBR-Adv3(Adv7-1)-EGFP, PBR-Adv3(Adv7-2)-EGFP, PBR-Adv3(Adv7-5)-EGFP, PBR-Adv3(Adv7-7)-EGFP, Among them, PBR-Adv3(Adv7-1)-EGFP means that hypervariable region 1 of Adv3 is replaced by hypervariable region 1 of Adv7, and so on.
[0036] Transfect cells with the positive plasmids obtained from the above recombination to obtain recombinant viruses MH1, MH2, MH5 and MH7; wherein recombinant virus MH1 is...
Embodiment 3
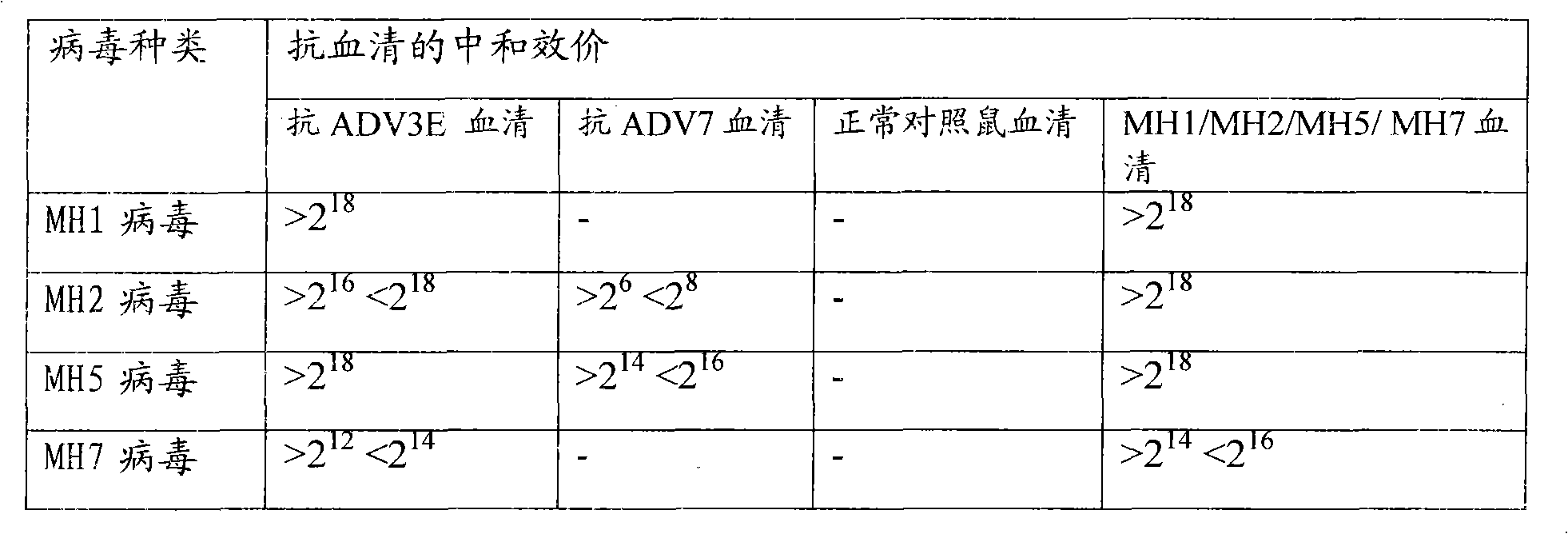
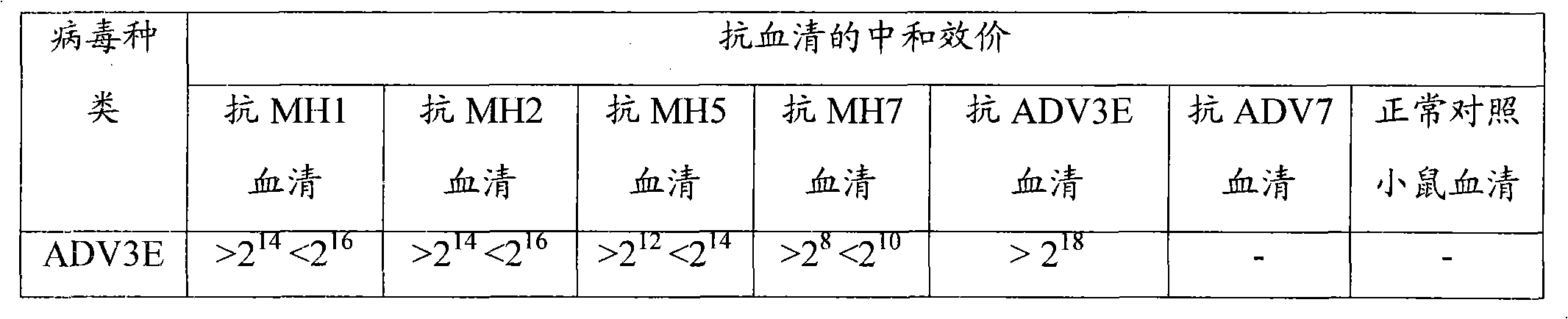
[0037] Embodiment 3: Serum neutralization test
[0038]To prepare antiserum, a serum neutralization test was performed. The purified recombinant viruses MH1, MH2, MH5 and MH7, wild type 3 ADV virus (ADV3E) and wild type 7 ADV virus (ADV7) with enhanced green fluorescent protein (EGFP) were injected intraperitoneally into Balb / C mice, respectively. The polyclonal antibodies of MH1, MH2, MH5 and MH7 as well as ADV3E and ADV7 were obtained respectively, and then the neutralization test was carried out. The neutralization test was divided into two groups: the first group was to detect the neutralization reaction between the anti-ADV3E and ADV7 serum and the recombinant virus MH1, MH2, MH5 and MH7, in order to detect whether the neutralizing antigen expression of ADV3 and ADV7 was included in the recombinant virus The second group is to detect the neutralization reaction between the antisera of MH1, MH2, MH5, MH7, ADV3E, ADV7 and normal control mice and ADV3, to detect whether the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com