Neutralizing monoclonal antibody in human adenovirus 7 and preparation method and application thereof
A monoclonal antibody, adenovirus technology, applied in microorganism-based methods, biochemical equipment and methods, antibodies, etc., can solve the difficulty of neutralizing monoclonal antibodies, difficult to show spatial conformation, and the virus cannot be cultured in cell lines. Yield and other problems to achieve the effect of enhancing the immune response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Construction of type 3 adenovirus vector Ad3EGFP
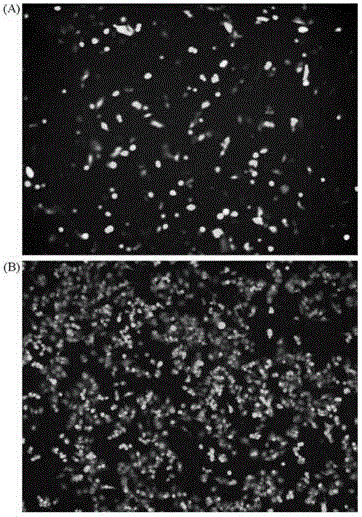
[0045] The adenovirus vector constructed in the present invention is based on the deletion of the human HAdv3 E3 region and the expression of the enhanced green fluorescent protein, and can express the recombinant adenovirus of the HAdv3 genome and the enhanced green fluorescent protein. The specific construction steps are as follows: after the HAdv3GZ01 virus strain was cultured in HEp-2 cells, the virus genome was extracted, and homologously recombined with the shuttle plasmid pBRALR in BJ5183 bacteria to obtain the plasmid pBRAdV with the full genome of the HAdv3GZ01 virus; after RsrII digestion and linearization of the plasmid pBRAdV Homologous recombination with the shuttle plasmid pSKE3LCMVeGFP-SV40R in BJ5183 bacteria, the recombinant plasmid pBRAd△E3GFP inserted into the EGFP gene expression cassette was obtained, and the recombinant adenovirus rAd△E3GFP was rescued after the plasmid was transfected in...
Embodiment 2
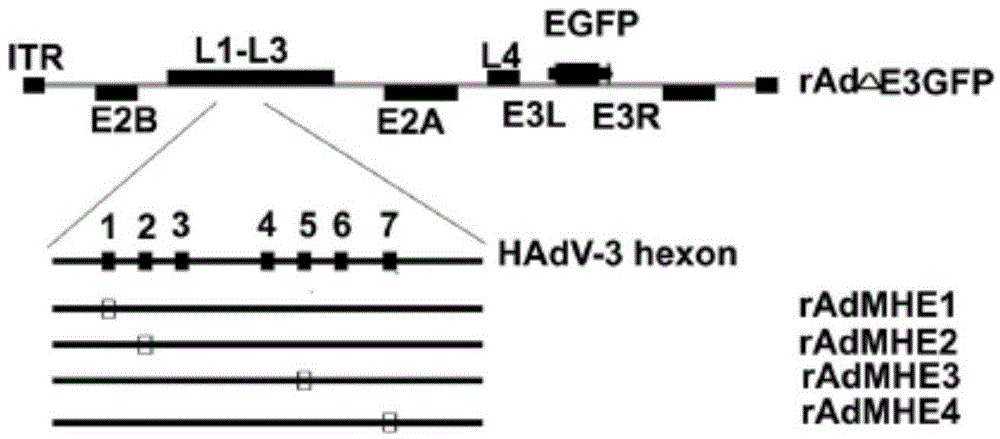
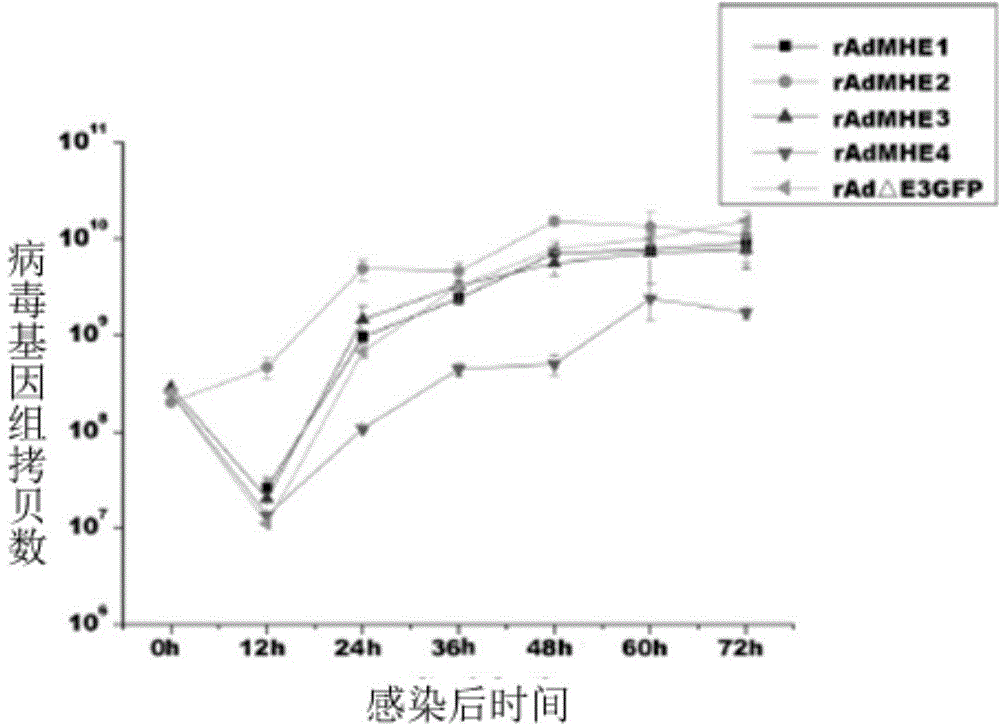
[0047] Example 2: Construction of immunogen rAdMHE3
[0048] The hexon hypervariable region 5 (HVR5) of Ad3EGFP ( 264 FDGRDAVAGALAPEIV 279 ) (the amino acid sequence of the 5th hypervariable region of the hexon of the human type 3 adenovirus vector is shown in SEQ NO: 2, and the sequence is located at the 264th to the 264th position of the hexon of the human type 3 adenovirus vector 279 bits) replaced by HAdv7's HVR5 ( 255 FDGREAADAFSPEIV 269 ) (the amino acid sequence of the 5th hypervariable region of the hexon of human adenovirus type 7 is shown in SEQNO: 1, and the sequence is located at the 255th to 269th position of the hexon of the human adenovirus type 7) , by-pass PCR to amplify the hexon gene of human type 3 cells mutated in the HVR5 region, digest and connect to the shuttle vector pBRLR, and homologously recombine with the vector pBRAd△E3GFP in BJ5183 bacteria to obtain the hexon-mutated recombinant adenoviral plasmid pBRAd- MHE3, rescue of recombinant virus rAd...
Embodiment 3
[0050] Embodiment 3: Identification uses the construction of chimeric virus rAdH7R1, rAdH7R2, rAdH7R5 and rAdH7R7
[0051] First construct rAd3egf / H7 (Ad3 / H7) chimeric virus, rAd3egf / H7 (Ad3 / H7) chimeric virus is a chimeric virus produced by replacing the hexon gene of Ad3EGFP with the hexon gene of HAdv7GZ08. Construction method: PCR amplified the hexon fragment of HAdv7GZ08 virus strain, connected to the shuttle plasmid pBRALR, homologously recombined with the pBRAd△E3GFP backbone plasmid in BJ5183 to obtain the recombinant plasmid pAd3egf / H7, and rescued and recombined after transfecting 293 cells Virus rAd3egf / H7. The neutralizing antibody titer experiment was carried out on the virus-immunized mice, and the experimental results are shown in Table 1. Table 1 is the result table of the neutralizing antibody titer experiment on the adenovirus-immunized mice. It can be seen from the data in Table 1 that the hexon in the virion is the hexon protein of adenovirus type 7, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com