Adenovirus bivalent vaccine
A technology of adenovirus and virus particles, applied in the field of virus immunology, can solve problems such as difficulty in production, achieve the effects of preventing infection, improving safety and application range, and improving replication ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
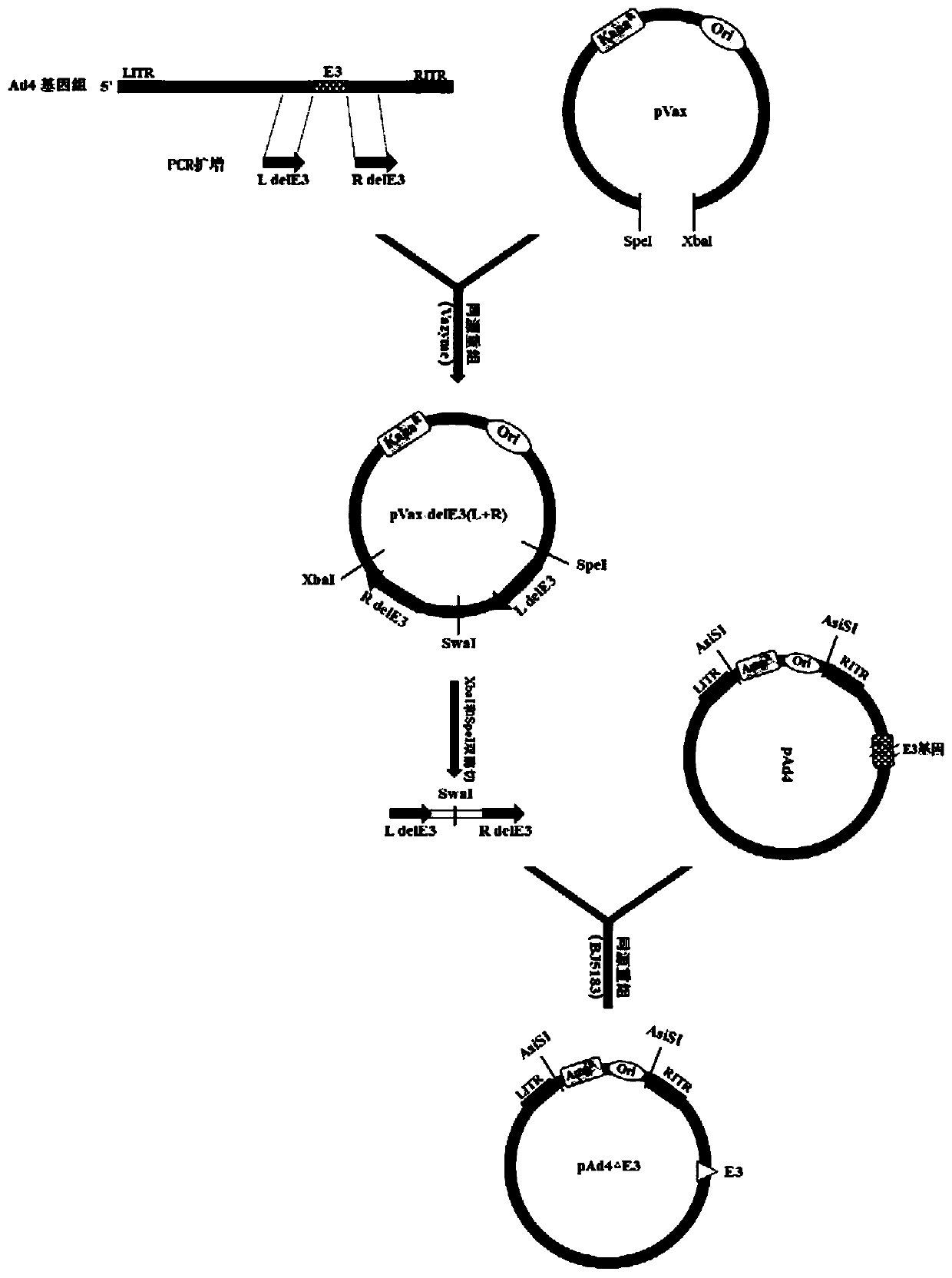
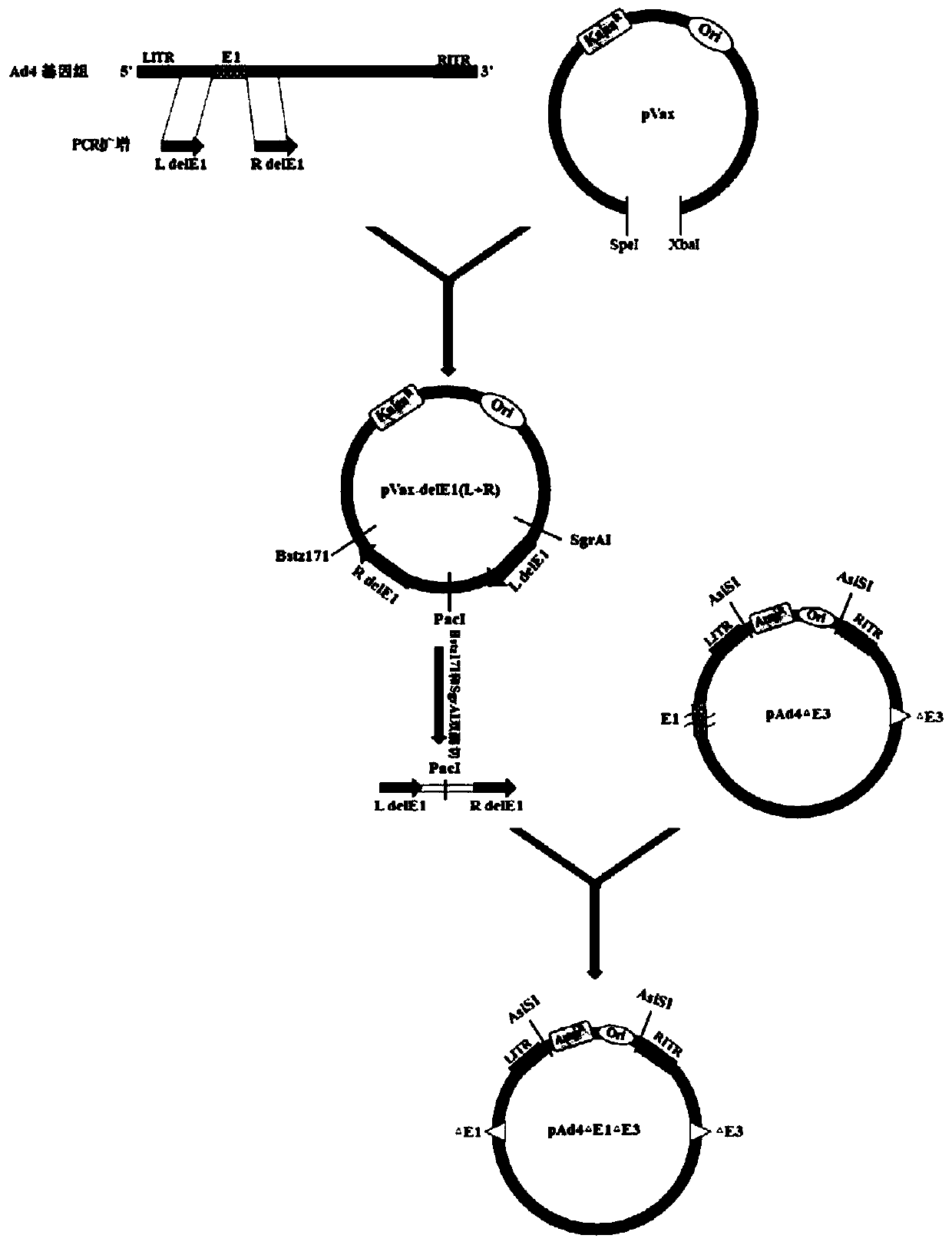
[0042] Preparation of Example 1 Replication-deficient Ad4 Vaccine
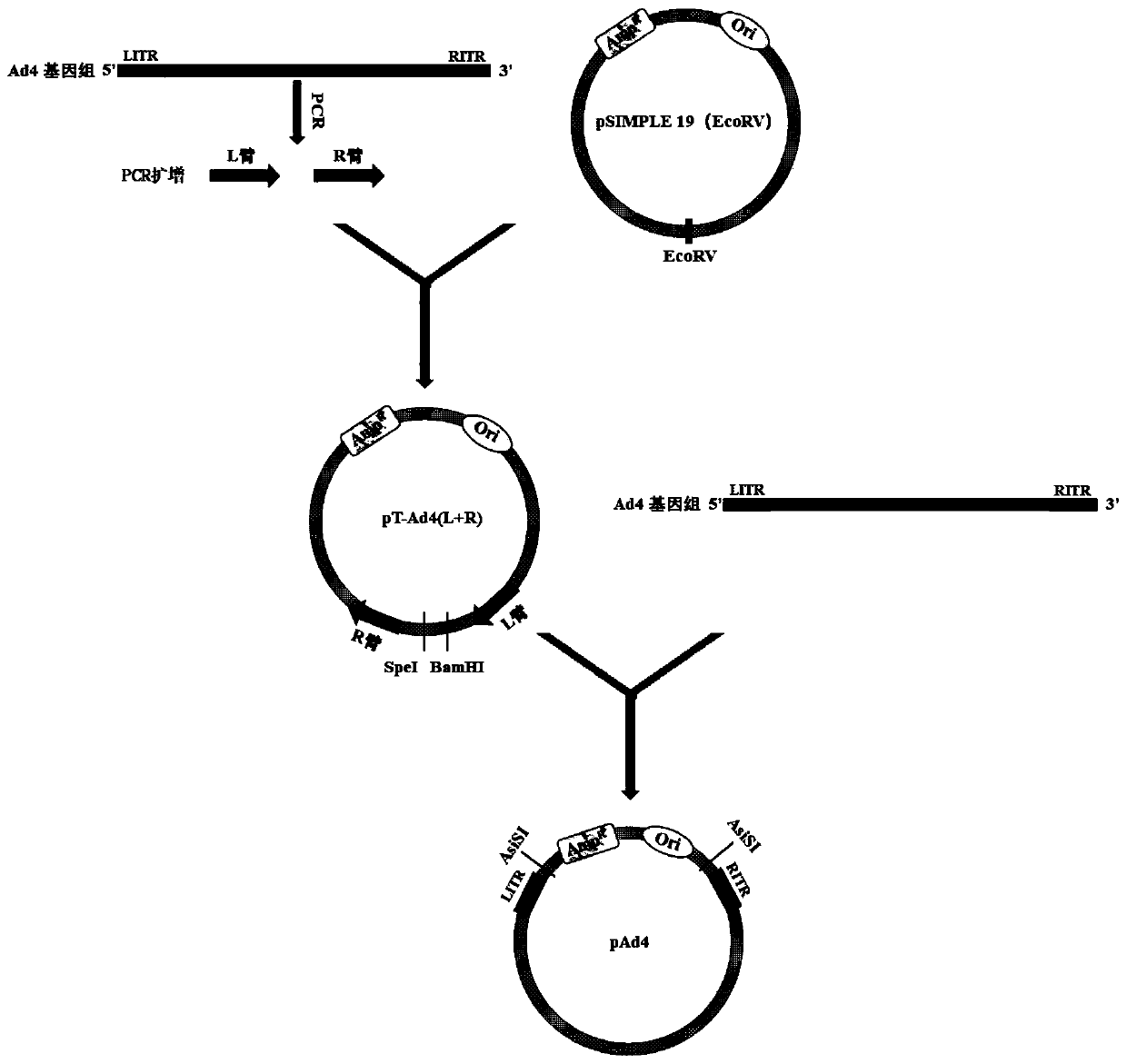
[0043] 1. Construction of the Ad4 genome circularization shuttle vector
[0044] 1. Construction of Ad4 genome circularization shuttle vector.
[0045]The Ad4 genome was used as a template for PCR amplification to obtain recombinant arms Ad4-L and Ad4-R.
[0046] Ad4-L primer sequence:
[0047] Ad4-L Fw, ATAGAATTCGGGGTGGAGTGTTTTTGCAAG (SEQ ID NO. 1);
[0048] Ad4-L Rw, TTTACTAGTGTTTAAACGTAATCGAAACCTCCACGTAATGG (SEQ ID NO. 2).
[0049] PCR program: 95°C, 30 seconds; 62°C, 30 seconds; 72°C, 20 seconds; 25 cycles.
[0050] Ad4-R primer sequence:
[0051] Ad4-R Fw, ACTAGTAGCTGGATCCAAGCCTCGAGGCACTACAATG (SEQ ID NO. 3);
[0052] Ad4-R Rw, CCTGCCGTTCGACGATGCGATCGCCATCATCAATAATATACCTTATAGATGG (SEQ ID NO. 4).
[0053] PCR program: 95°C, 30 seconds; 55°C, 30 seconds; 72°C, 80 seconds; 25 cycles.
[0054] The Ad4 genome circularization shuttle plasmid pT-Ad4(L+R) was obtained by ligation with pSIMPLE 19 (EcoRV) v...
Embodiment 2
[0132] Preparation of Example 2 Replication Deficient Ad7 Vaccine
[0133] 1. Circularization of the Ad7 genome
[0134] 1. Construction of the shuttle plasmid pT-Ad7(L+R) for circularizing the Ad7 genome.
[0135] Using the Ad7 genome as a template, the left arm (L-Ad7) and the right arm (R-Ad7) of the Ad7 genome were obtained by PCR.
[0136] L-Ad7 primer:
[0137] L-Ad7-F: ACTGCGATCGCCTCTCTATTTAATATACCTTATAGATGG (SEQ ID NO. 25);
[0138] L-Ad7-R: ACATGGATCCTCACTGAAGATAATCTCCTGTGG (SEQ ID NO. 26).
[0139] PCR conditions: 95°C, 3min; 95°C, 30s; 56°C, 30s; 72°C, 40s; cycles 30; 72°C, 5min;
[0140] R-Ad7 primer:
[0141] R-Ad7-F: AGCTGGATCCGAACCACCAGTAATATCATCAAAG (SEQ ID NO. 27);
[0142] R-Ad7-R: TGAGCGATCGCCTCTCTATATAATATACCTTATAGATGGAA (SEQ ID NO. 28).
[0143] PCR conditions: 95°C, 3min; 95°C, 30s; 56°C, 30s; 72°C, 1min; cycles 30; 72°C, 5min;
[0144] The PCR product and the T vector were ligated with three fragments using Exnase recombinase to obtain pT-Ad7(L+R...
Embodiment 3
[0221] Embodiment 3 Ad4 and Ad7 bivalent vaccine preparation
[0222] 1. Vaccine storage
[0223] The replication-defective Ad4 and Ad7 vaccines purified by cesium chloride density gradient force centrifugation were carried out until the concentration of Ad4 was 4×10 11 vp / ml, the concentration of Ad7 is 4×10 11 vp / ml, stored at -80°C.
[0224] 2. Immunogenicity evaluation of Ad4 and Ad7 bivalent vaccine in macaques
[0225] The immunogenicity evaluation scheme of Ad4 and Ad7 quadrivalent vaccine in macaques was designed, as shown in Table 1, and the immunogenicity of Ad4 and Ad7 bivalent vaccine was evaluated according to the designed immunization scheme. Ad4 and Ad7 bivalent vaccine (Ad4: 2×10 10 vp / ml, Ad7: 2×10 10 vp / ml).
[0226] Table 1
[0227]
[0228] Adult rhesus monkeys were selected and divided into 2 groups with 4 monkeys in each group. Intramuscular injection (arm) was adopted for immunization. The first group was immunized with Ad4 and Ad7 bivalent va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com