Inhibition of adenoviruses with felociclovir
An adenovirus, a technology of uses, applied in the direction of antiviral agents, medical preparations containing active ingredients, peptide/protein ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
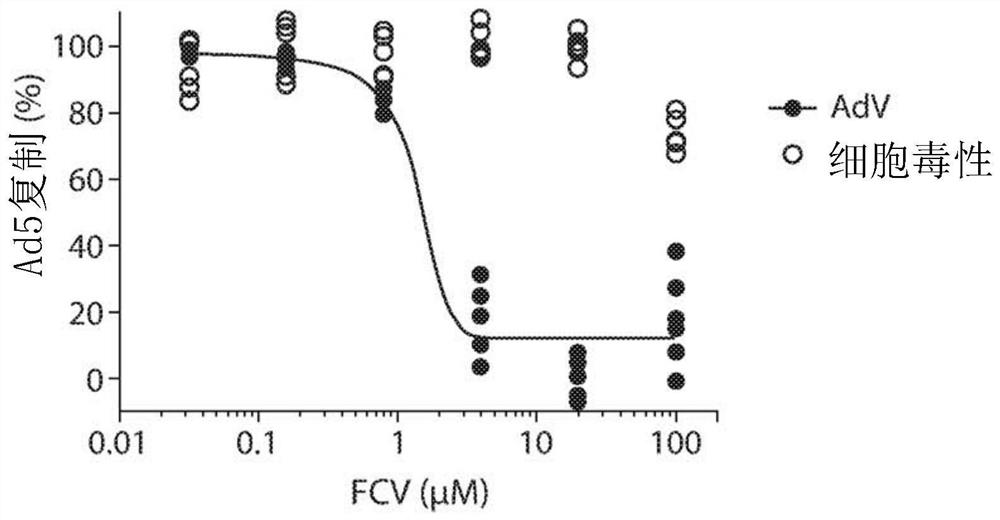
[0083] Example 1. Cytopathic Effect (CPE) Reduction Assay to Measure Felociclovir Inhibition of Adenovirus 5 (AdV5)
[0084] Preparation and culture of human foreskin fibroblast (HFF) cells
[0085] Human foreskin tissue was obtained from the University of Alabama tissue procurement facility at Birmingham and was approved by the Institutional Review Board. Tissues were stored at 4°C in cell culture medium consisting of Minimal Essential Medium (MEM) with Earle's salts supplemented with 10% fetal bovine serum (FBS; HyClone, Inc., Logan, UT), and standard Concentrations of L-glutamine, amphotericin B (Fungizone) and vancomycin. The tissue is then placed in a phosphate-buffered saline solution, minced and rinsed to remove red blood cells. The tissue fragments were then resuspended in trypsin-EDTA solution and incubated at 37°C to disperse the cells, which were then collected by centrifugation. The cell pellet was then resuspended in 4ml medium and placed at 25cm 2 tissue cu...
Embodiment 2
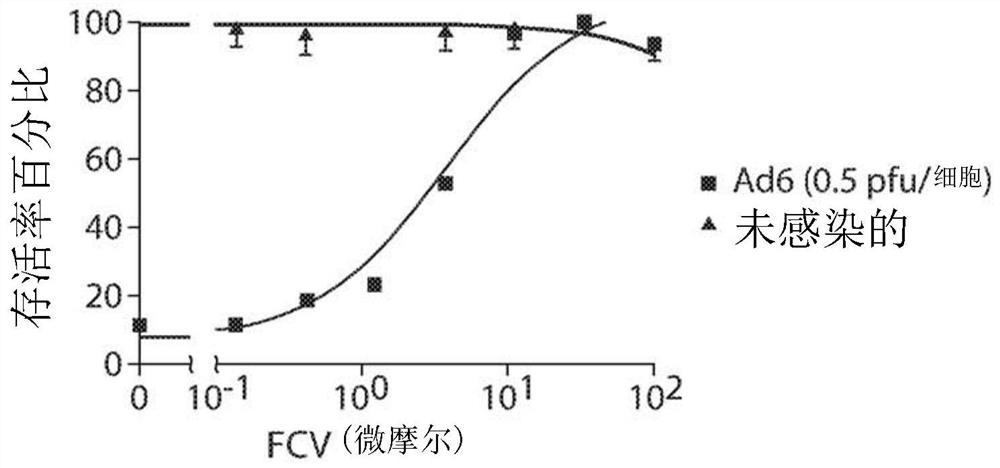
[0102] Example 2. Neutral Red Survival Assay to Measure Felociclovir Inhibition of Adenovirus 6 (AdV6)
[0103] Neutrophil cytotoxicity assays are used to detect cell viability or drug cytotoxicity. The principle of the assay is based on the detection of living cells via the uptake dye neutral red. Neutral red is a diamine azine (eurhodine) dye that can stain lysosomes in living cells. Viable cells can take up Neutral Red and incorporate the dye into their lysosomes via active transport, but nonviable cells cannot take up this chromophore. Thus, after washing, living cells can release the incorporated dye under acidified extraction conditions. The amount of dye released can be quantified and used to determine the total number of viable cells or the cytotoxicity of a drug. Thus, cytotoxicity is expressed as a concentration-dependent decrease in the uptake of neutral red following exposure to the investigated compounds.
[0104] The neutral red survival assay was performed i...
Embodiment 3
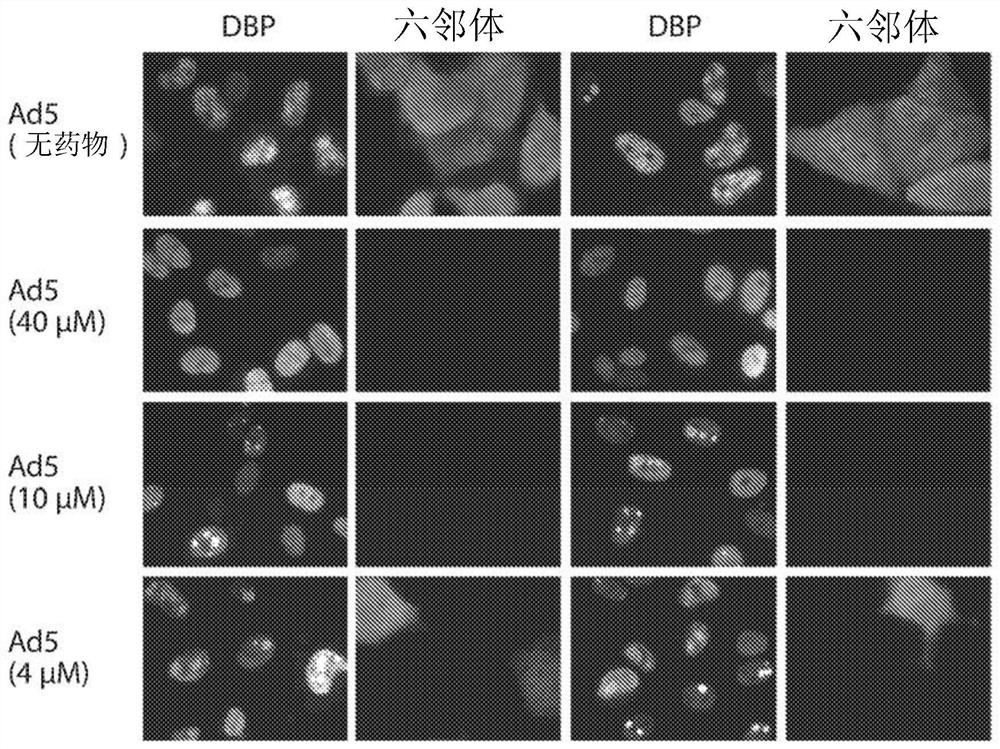
[0106] Example 3. Immunofluorescence assay of human A549 cells infected with AdV5 or AdV6 treated with felociclovir
[0107] Human A549 cells on glass coverslips in 6-well plates were infected or mock-infected with AdV5 or AdV6 at an MOI of 5 PFU / cell. After 1 hour, felociclovir was added to a final concentration of 0, 4, 10 or 40 [mu]M. Twenty-seven hours after infection, A549 cells were fixed in paraformaldehyde (3.7% in PBS) and permeabilized with methanol. Cells were stained for adenovirus DNA binding protein and adenovirus hexon. Early in AdV infection (before AdV DNA replication), DBP staining will be antinuclear and uniform. As the infection progresses, DBP associates with replication centers. Centers of replication are initially small "dots" (each from an incoming AdV genome). As DNA replication occurs, the replication centers will amplify and "multiply." As the infection progresses, the nuclei become enlarged and deformed. The AdV hexon, the most abundant compon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com