Culturing method of adenovirus gene-modifying tumor specificity cytotoxic T lymphocyte
A tumor-specific and cytotoxic technology, applied in the field of tumor-specific cytotoxic T lymphocyte culture, can solve the problems of patient influence, low tumor cell targeting, low antigen specificity, etc. Therapeutic effect, the effect of reducing the burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Obtaining mononuclear cells from cord blood or autologous peripheral blood
[0063] ①Wipe the blood collection bag with dust-free paper soaked in alcohol and put it into the biological safety cabinet. Use a 50ml syringe to draw the blood in the blood bag and divide it into 50ml centrifuge tubes. Each tube has 30ml of blood. Centrifuge for 10 minutes;
[0064] ② After centrifugation, put the upper layer plasma into a new 50ml centrifuge tube. Suction until 0.5cm away from the interface, if the remaining blood cells are more than 15ml, take the upper 15ml of blood cells and place them in a new centrifuge tube, discard the remaining red blood cells at the bottom;
[0065] ③Dilute the white blood cells with normal saline at a ratio of 1:1, take a centrifuge tube containing 15ml of ficoll and tilt it, and slowly add the mixed blood cells to the surface of the ficoll to make it a clear interface. The ratio of blood sample to ficoll is not the same. More than 2:1,...
Embodiment 2
[0069] Example 2: A method for obtaining immature DCs from umbilical cord blood mononuclear cells induced by cytokines
[0070] ①Set the cell density to 10 7 / ml of umbilical cord blood mononuclear cells according to 10 6 / cm 2 The number of cells added to the culture flask, 37 ° C, 7.5% CO 2 Cultivate in a concentration incubator;
[0071] ② After standing for 2 hours, gently shake the culture bottle to shake up the non-adhered cells, absorb the upper layer of non-adhered cells and transfer them to a new culture bottle; add X-VIVO15 containing GMCSF1000U / ml and IL-41000U / ml Medium, at 37°C, 7.5% CO 2 Cultivate in a concentration incubator;
[0072] ③The next day, shake the culture bottle to shake up the non-adherent cells, discard the culture medium, and refill the X-VIVO15 medium containing GMCSF1000U / ml and IL-41000U / ml to continue the culture;
[0073] ④Change the medium every two and a half days, add X-VIVO15 medium containing GMCSF1000U / ml and IL-41000U / ml, complet...
Embodiment 3
[0074] Example 3: A method for inducing maturation of immature DCs by adenovirus infection
[0075] ①Collect the immature DC cultured to the 5th day, take a sample and count, calculate the total amount of DC, centrifuge at 1500rpm for 10min;
[0076] ② Discard the supernatant after centrifugation, add X-VIVO15 medium containing 1000U / mlGMCSF, 1000U / mlIL-4 and 100ng / ml TNF-α to resuspend the cells, and adjust the cell density to 5×10 6 / ml; According to the MOI is 10 4 Add the virus dosage of vp / cell to the cell suspension, mix thoroughly, spread in culture flasks, and store at 37°C, 7.5% CO 2 Cultivate in a concentration incubator;
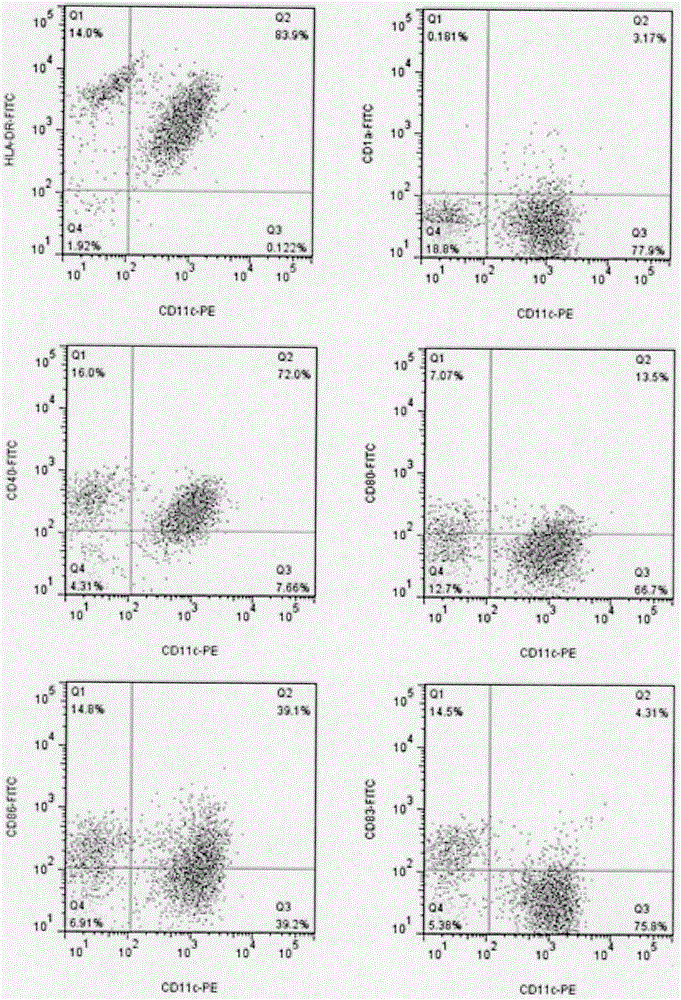
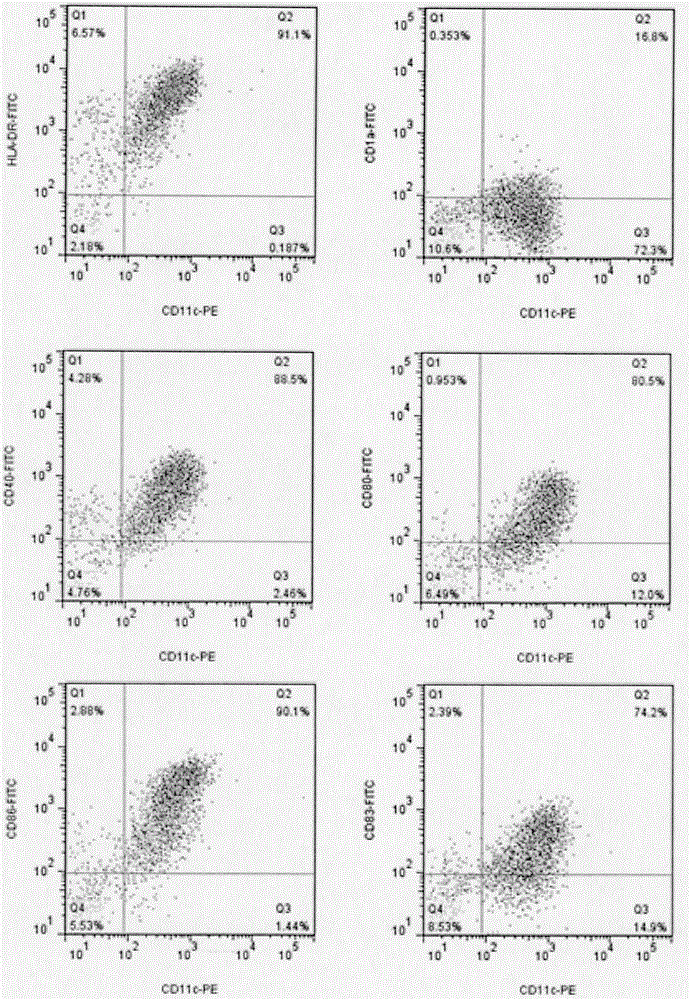
[0077] ③ After culturing for 48 hours, collect the suspended cells, scrape up the unsuspended cells with a cell scraper, and mix the two types of cells into mature DC cells. Sampling and counting, flow cytometry detection of cell phenotype;
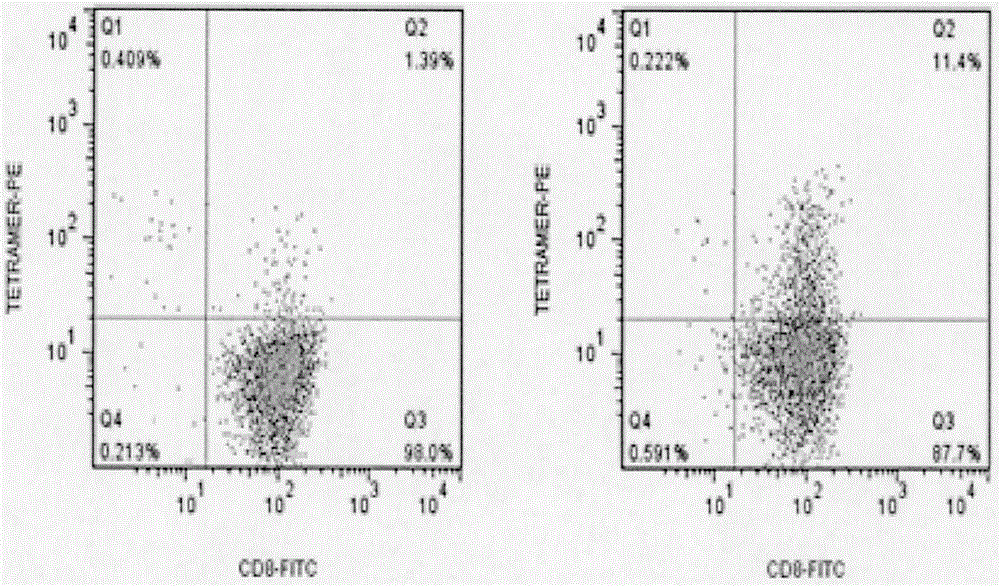
[0078] ④Determine the requirement of DC according to the amount of T cells, resuspend the remaining ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com