Method for constructing ribosome inactivating protein gene virus vector and expressing active proteins in tumor cell through ribosome inactivated protein gene virus vector
A technology for ribosome inactivation and tumor cells, which is applied in the construction of ribosome inactivation protein gene viral vectors and the expression of active proteins in tumor cells. problems, to achieve significant toxicological effects, significant cytotoxic effects, and significant effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
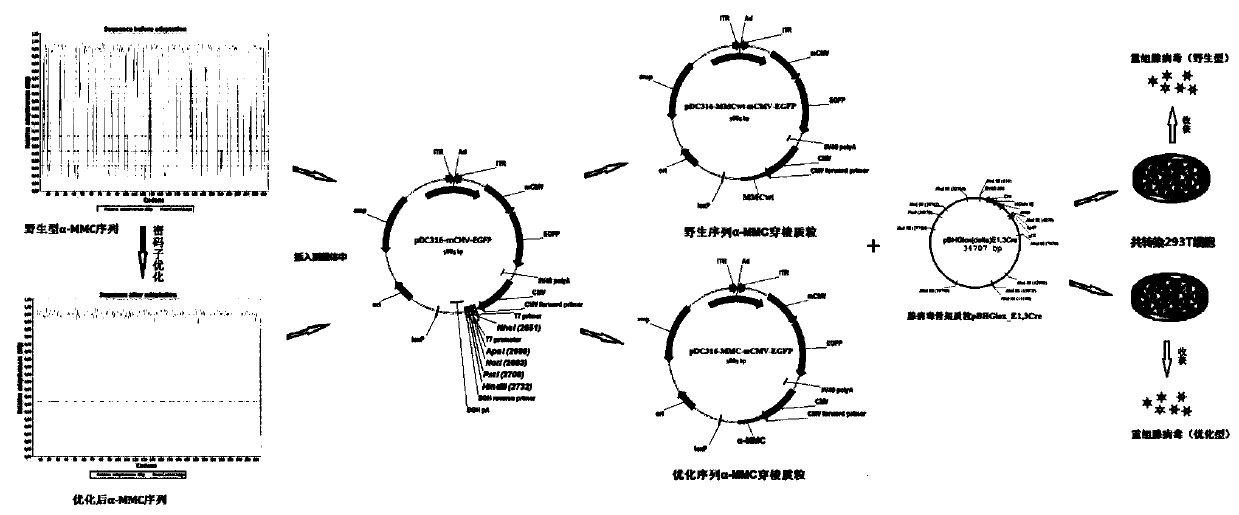
[0042] Step 1: Codon optimization of the alpha charantin gene
[0043] 1 Experimental method
[0044]Three professional codon optimization software were used to simultaneously optimize the mammalian codon of the mature region of alpha-momorcharin, combining the advantages of each software to obtain an ideal optimization result. The optimization process follows the following main principles: ① species codon usage preference, ② GC content, ③ CpG dinucleotide content, ④ cryptic splice sites, ⑤ mature PolyA sites in advance, ⑥ stagnation points and ribosome binding sites, ⑦ Negative strand CG island, ⑧RNA instability motif (ARE), ⑨repeated sequence, ⑩restriction enzyme cutting site. In addition, Kozak sequence and SD sequence are used to improve mRNA translation efficiency, and TGA stop codon is used to improve translation termination signal.
[0045] 2 Codon optimization results
[0046] 2.1 Analysis of amino acid expression rate before optimization
[0047] See figure 2 . ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com