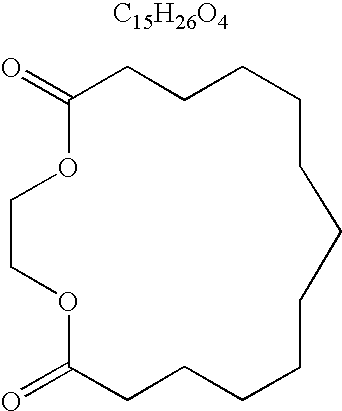
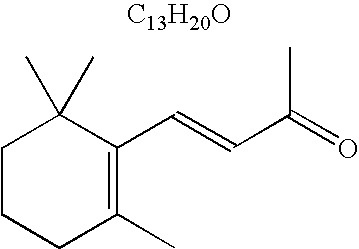
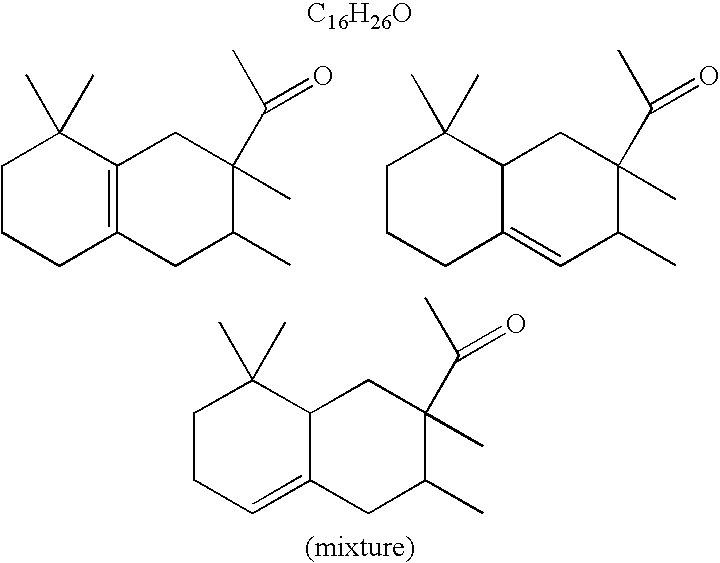
Methods of treating skin with aromatic skin-active ingredients
a technology of skin-active ingredients and aromatics, applied in the field of skin-active ingredients, can solve the problems of additional chemical ingredients and unpleasant odors of certain ingredients, and achieve the effects of reducing internal oxidation and/or external oxidative damage, reducing skin inflammation, and increasing collagen synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Efficacy Data for the Aromatic-Skin Active Ingredients
[0057]The following Tables 2-4 provide data confirming the efficacy of the aromatic skin-active ingredients identified in Table 1 above:
TABLE 2Aromatic Skin-ActiveCox-1Cox-2LOMMP3MMP9TNF-αIngredientInhibitionInhibitionInhibitionInhibitionInhibitionInhibition(alternate name)(0.020%)(0.001%)(0.1%)(0.001%)(0.001%)(0.01%)Aldehyde C-11−38.68%MOA2,4-nonadien-1-al−21.15%−23.75%−19.87%2-dodecenal (E)−24.98%Aldehyde C-12−27.64%LauricBeta Ionone−19.82%2,6-nonadien-1-al−11.42%−17.58%−45.58%−20.34%−32.53%Vertenex ® H.C.−18.50%−37.86%Neofolione−27.46%−53.90%Precyclemone B−25.09%Cyclacet−26.37%Melanol−20.45%Floralozone−33.20%Calone 1951−21.26%
TABLE 3Aromatic Skin-Active IngredientCox-2 InhibitionMMP9 Inhibition(alternate name)(0.001%)(0.01%)Leaf Acetate−22.04%Dihydro-jasmone−32.33%−37.47%Romascone ®−23.31%Iso E super−20.80%Stemone−22.31%Polysantol−36.09%4-methyl-3-decen-5-ol−28.09%Iso Cyclo Citral−31.58%−21.78%3-hexen-1-ol (E) and (Z)−23.56%−2...
example 2
Assays to Assess Efficacy
[0058]The following assays were used to obtain the results identified in Tables 2-4.
[0059]Antioxidant (AO) assay: An in vitro bioassay that measures the total anti-oxidant capacity of compounds. The assay relies on the ability of antioxidants in the sample to inhibit the oxidation of ABTS® (2,2′-azino-di-[3-ethylbenzthiazoline sulphonate]) to ABTS®•+ by metmyoglobin. The antioxidant system of living organisms includes enzymes such as superoxide dismutase, catalase, and glutathione peroxidase; macromolecules such as albumin, ceruloplasmin, and ferritin; and an array of small molecules, including ascorbic acid, α-tocopherol, β-carotene, reduced glutathione, uric acid, and bilirubin. The sum of endogenous and food-derived antioxidants represents the total antioxidant activity of the extracellular fluid. Cooperation of all the different antioxidants provides greater protection against attack by reactive oxygen or nitrogen radicals, than any single compound alone...
example 3
Compositions
[0068]Non-limiting examples of compositions of the present invention that can include an aromatic skin-active ingredient are described in Tables 5 and 6.
TABLE 5*Ingredient% Concentration (by weight)Phase AWater84.44Xanthum gum0.1M-paraben0.15P-paraben0.1Citric acid0.01Phase BCetyl alcohol4.0Glyceryl stearate + PEG 1004.0Octyl palmitate4.0Dimethicone1.0Tocopheryl acetate0.2Phase C**Aromatic Skin-Active2.0Ingredient(s)*Procedure for making composition: Sprinkle Xanthum gum in water and mix for 10 min. Subsequently, add all ingredients in phase A and heat to 70-75° C. Add all items in phase B to separate beaker and heat to 70-75° C. Mix phases A and B at 70-75° C. Continue mixing and allow composition to cool to 30° C. Subsequently, add phase C ingredient while mixing.**The aromatic skin-active ingredients identified throughout this specification can be incorporated into this composition. Additionally, any combination of such ingredients (including 2, 3, 4, 5, 6, 7, 8, 9, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com