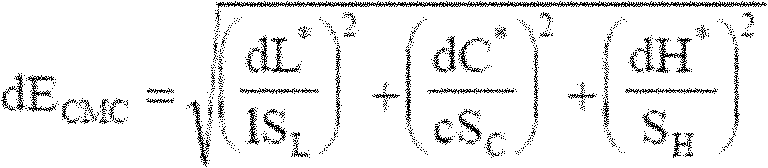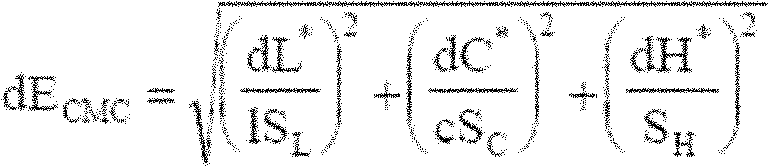Patents
Literature
55 results about "Skin Discoloration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
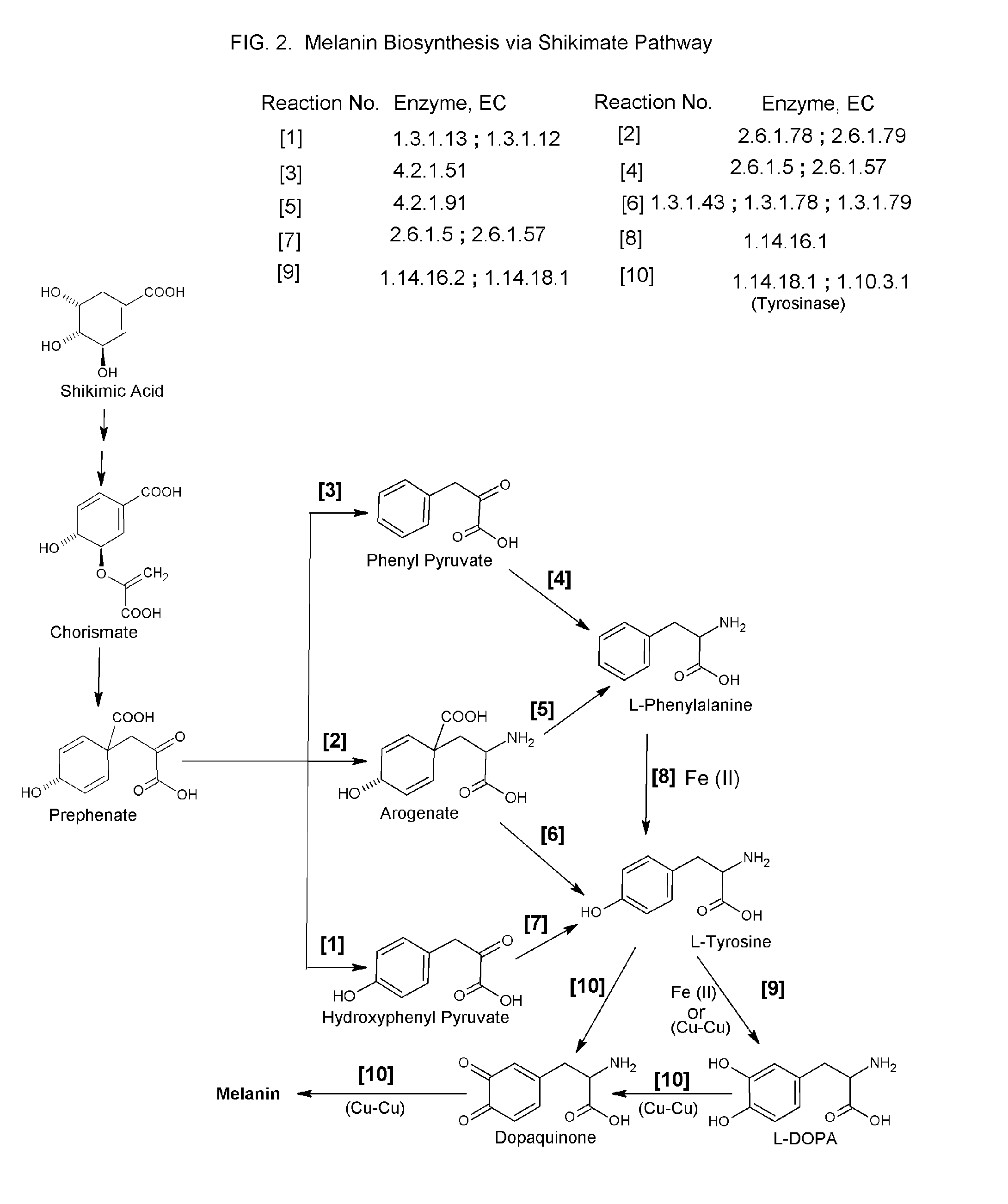
Abnormal changes in skin coloration.
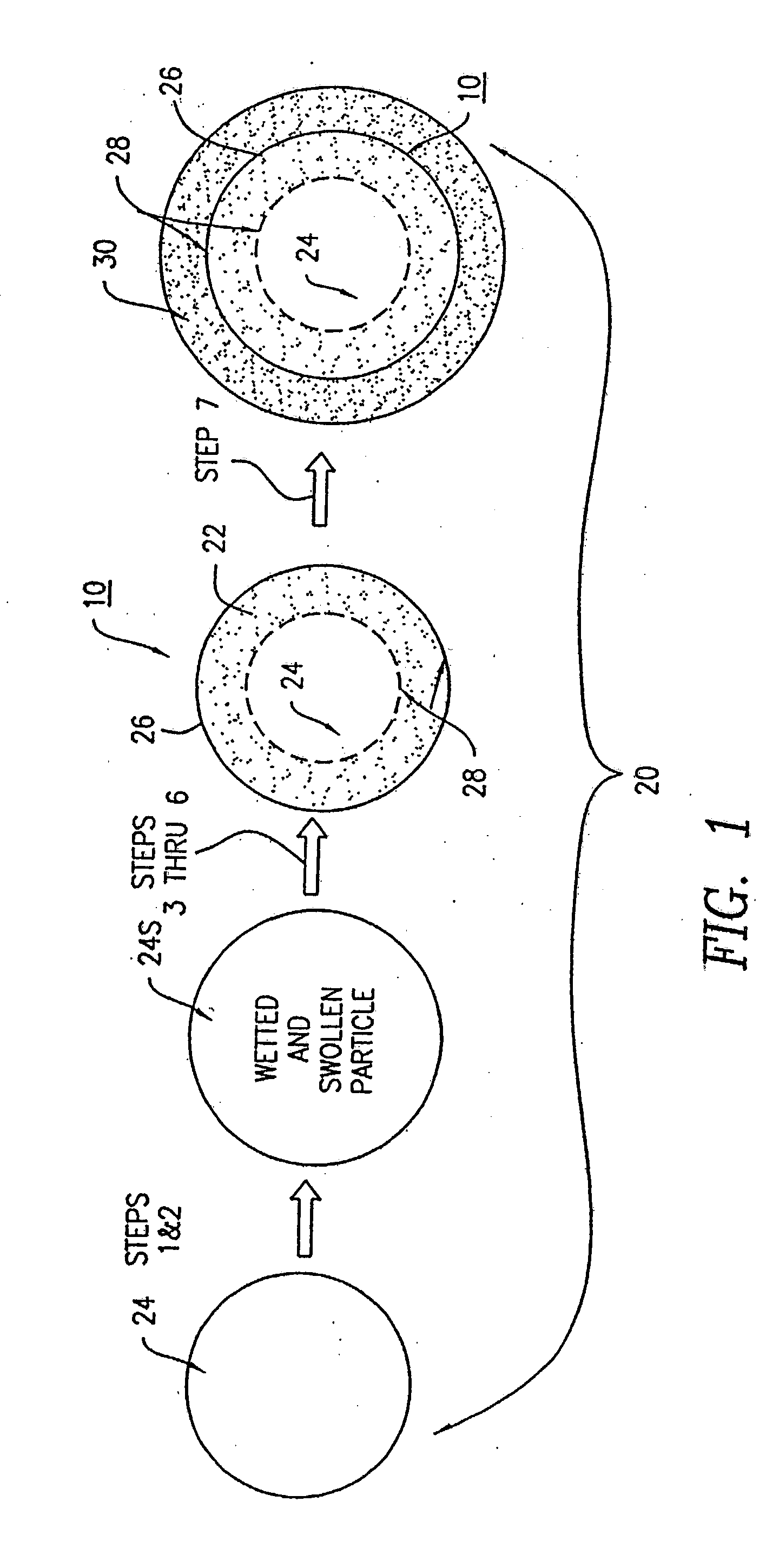
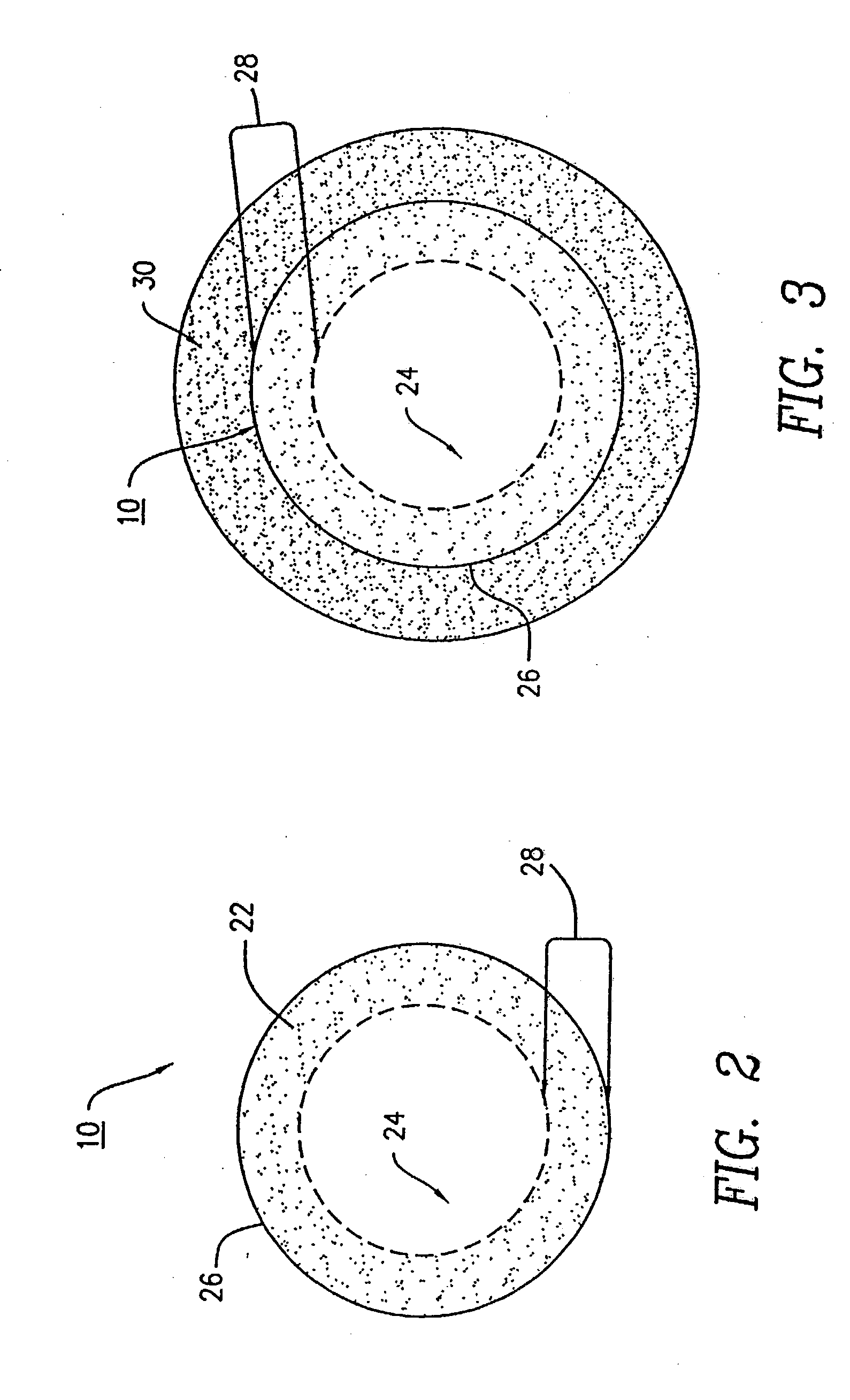
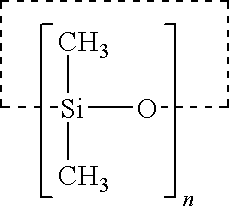
Stabilization of ascorbic acid, ascorbic acid derivatives and/or extracts containing ascorbic acid for topical use
InactiveUSRE38623E1Effective and stable mannerStable composition and pharmaceutical delivery systemBiocideCosmetic preparationsWrinkle skinSkin color
A stable composition is provided to treat and / or prevent photo-aged skin and related skin disorders such as sunburn, wrinkles, poor skin tone and skin discoloration by topically applying to the skin the treatment composition containing an effective amount of a compound such as ascorbic acid, derivatives of ascorbic acid and / or extracts containing ascorbic acid, in a pharmaceutically acceptable vehicle containing a substantially anhydrous base having no water added. The anhydrous base stabilizes the compound, so that the compound remains effective for an effective period of time, even in the presence of exposure to water.
Owner:TOPIX PHARMA INC
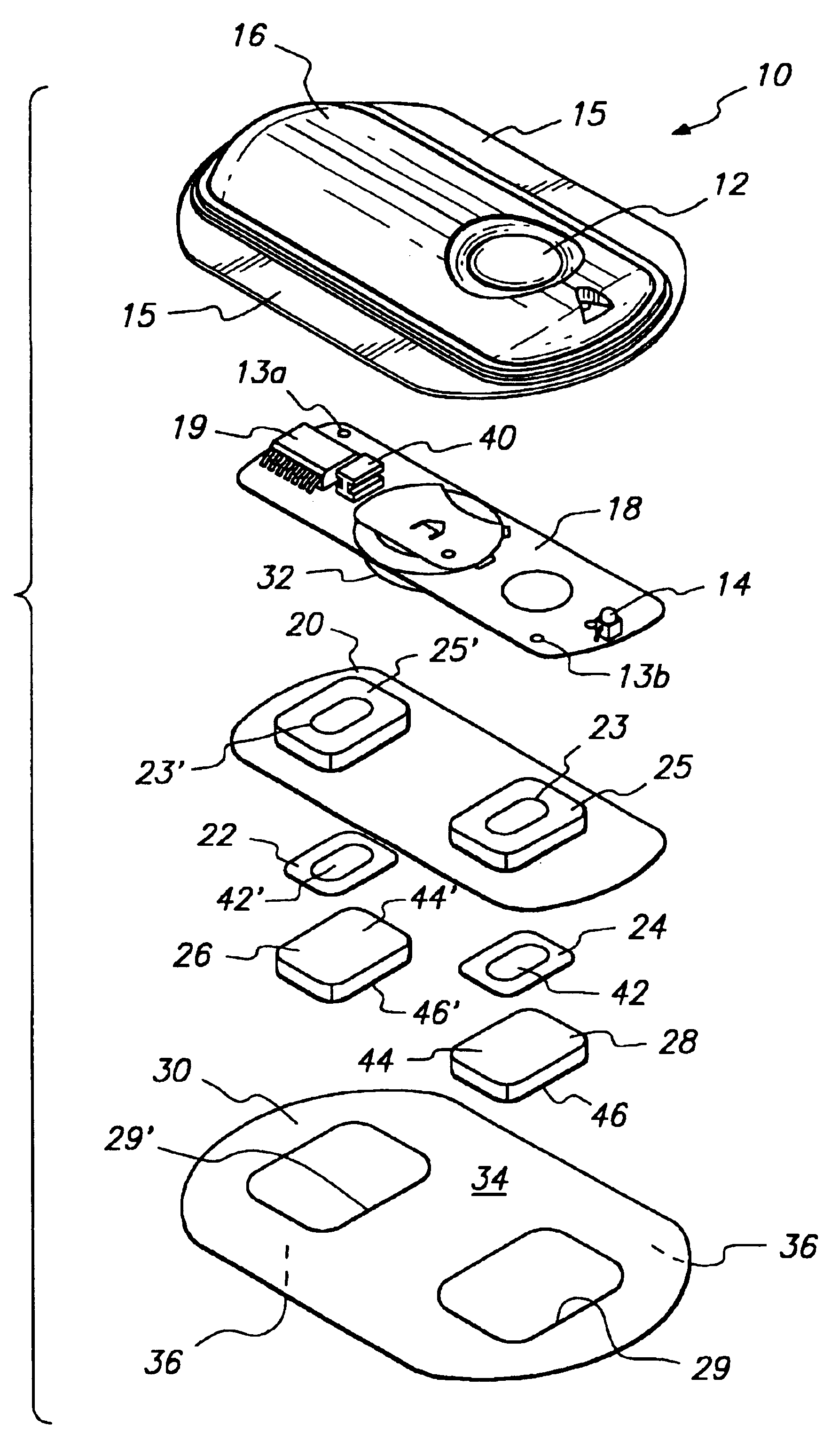
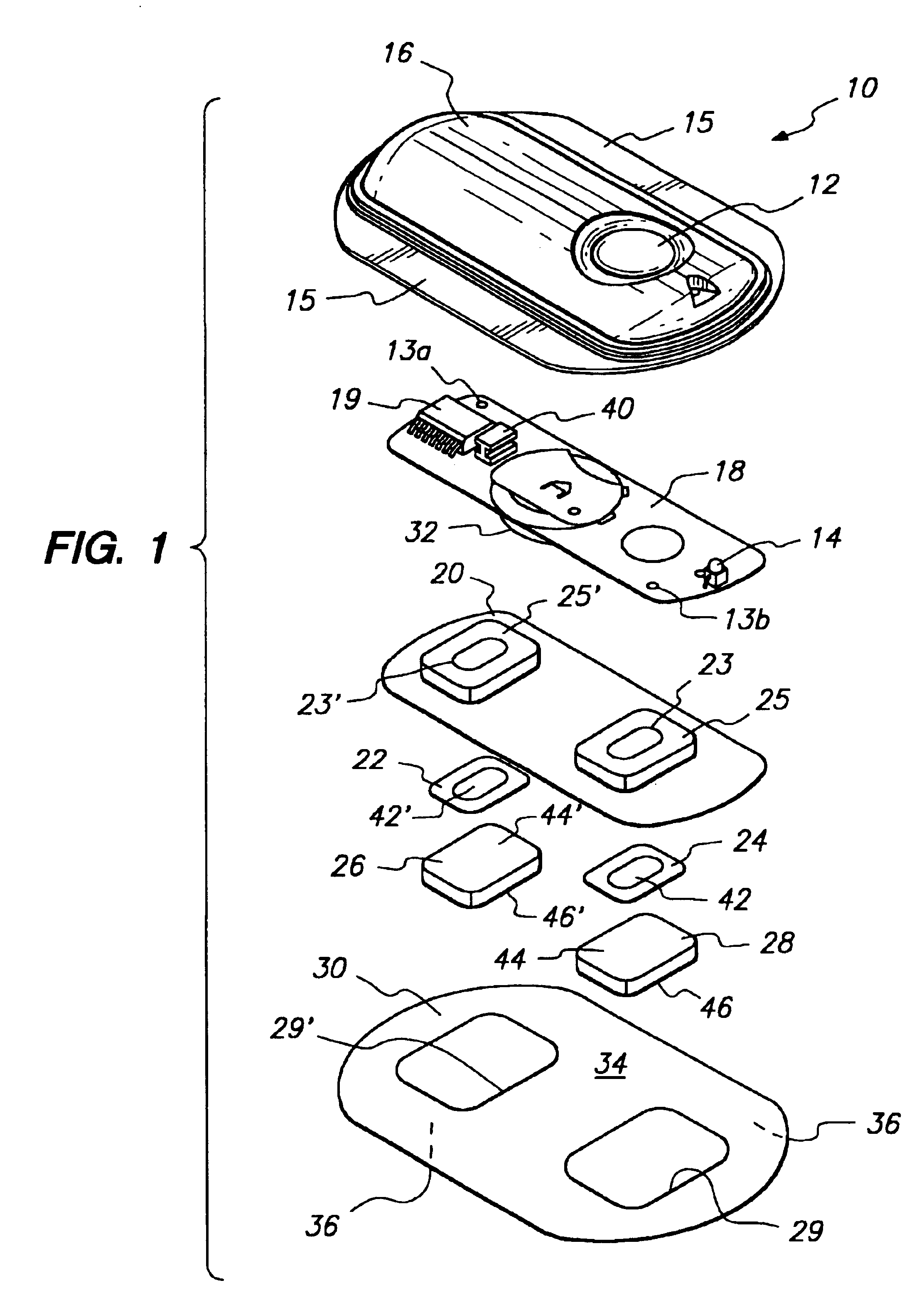
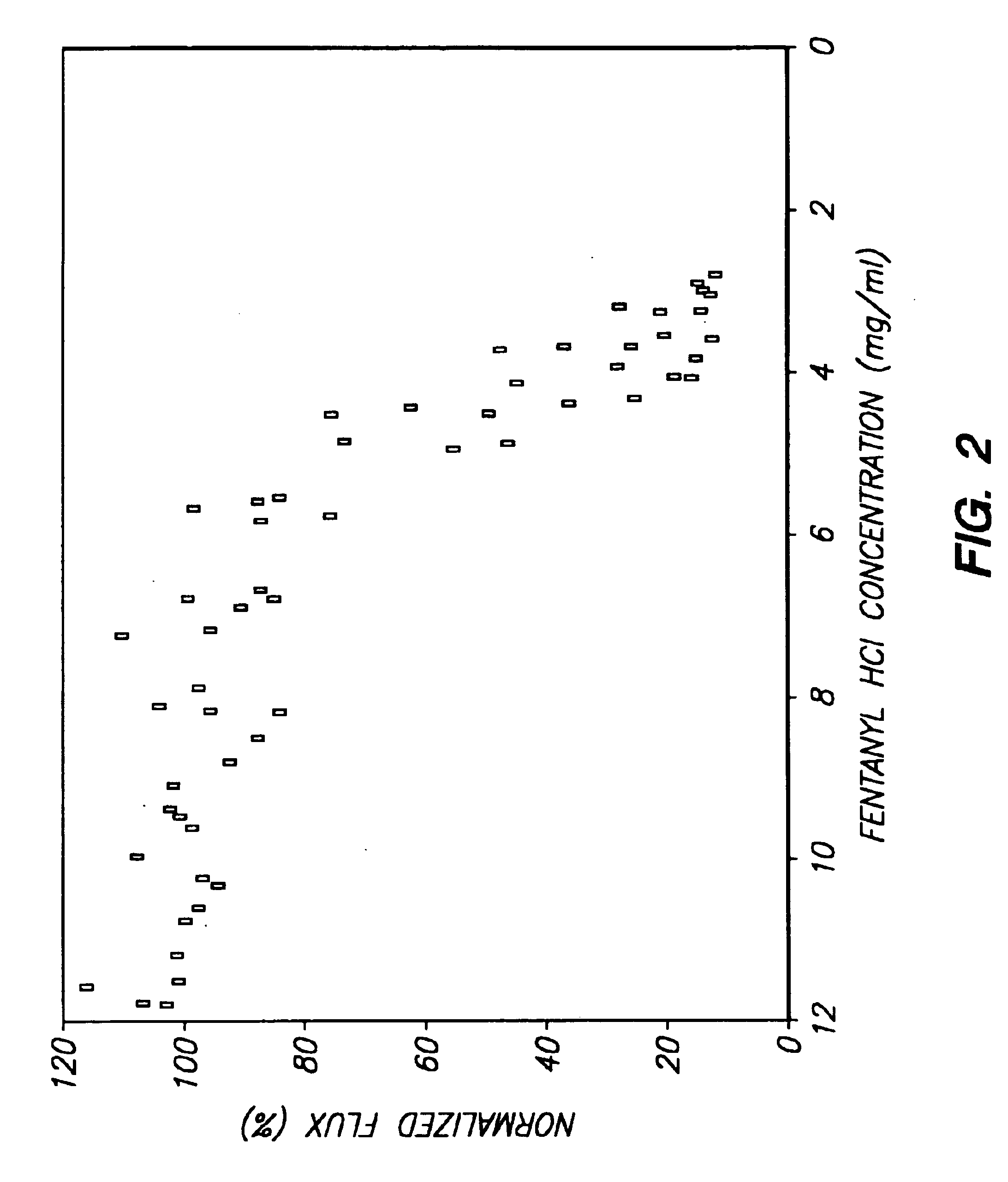
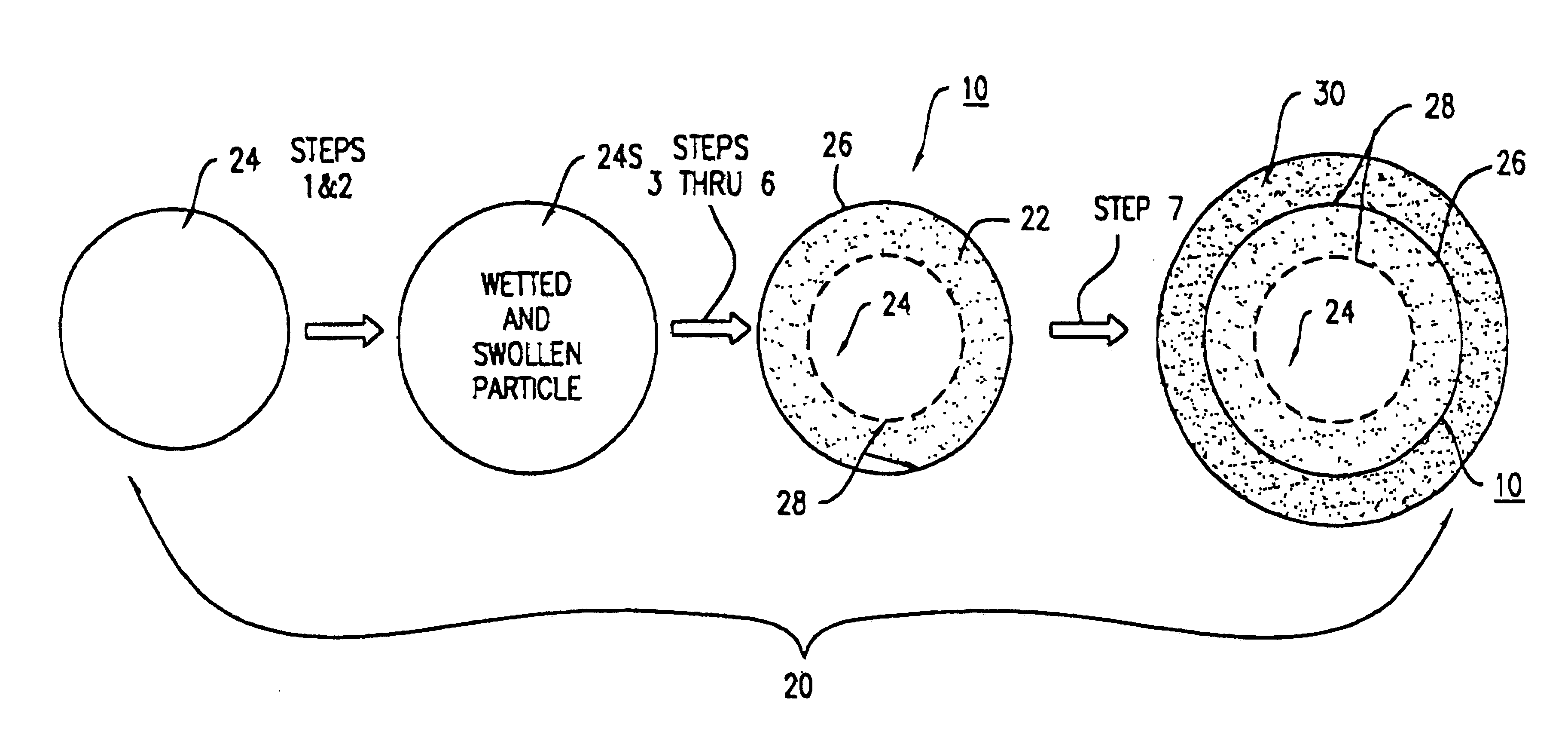
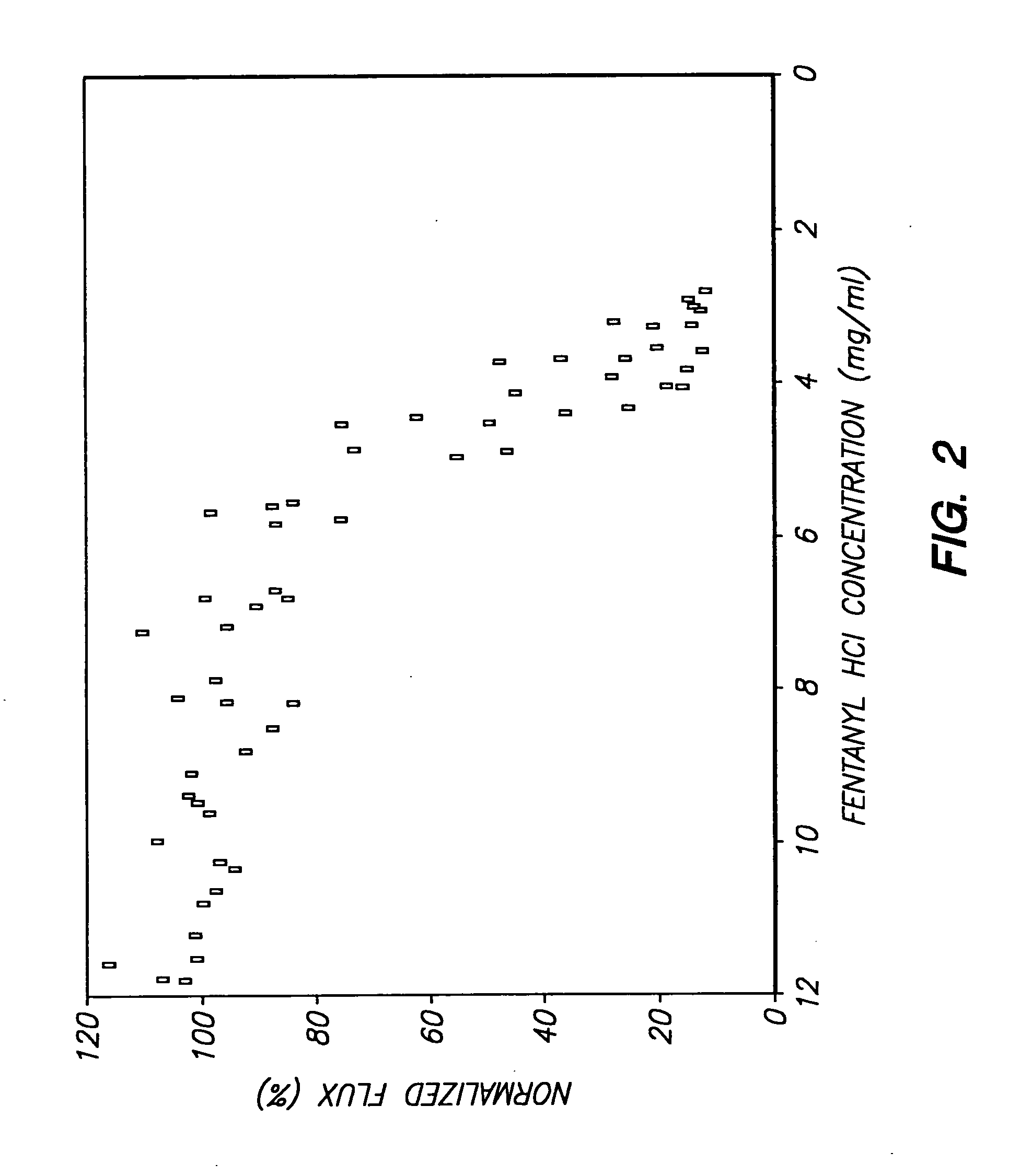
Method and device for transdermal electrotransport delivery of fentanyl and sufentanil
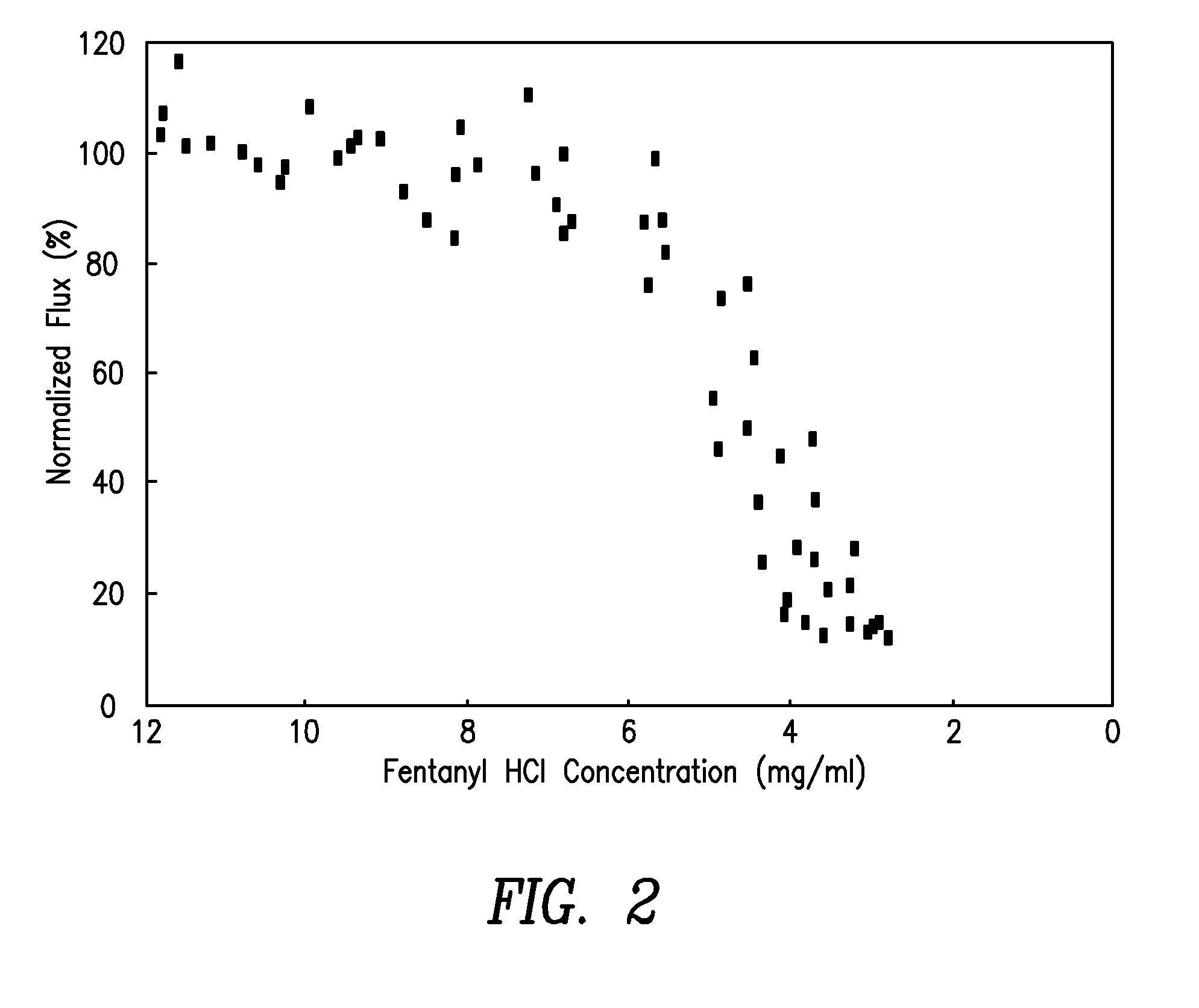
InactiveUS6881208B1Improved transdermal electrotransport deliveryImprove efficiencyOrganic active ingredientsNervous disorderAnalgesics drugsMedicine
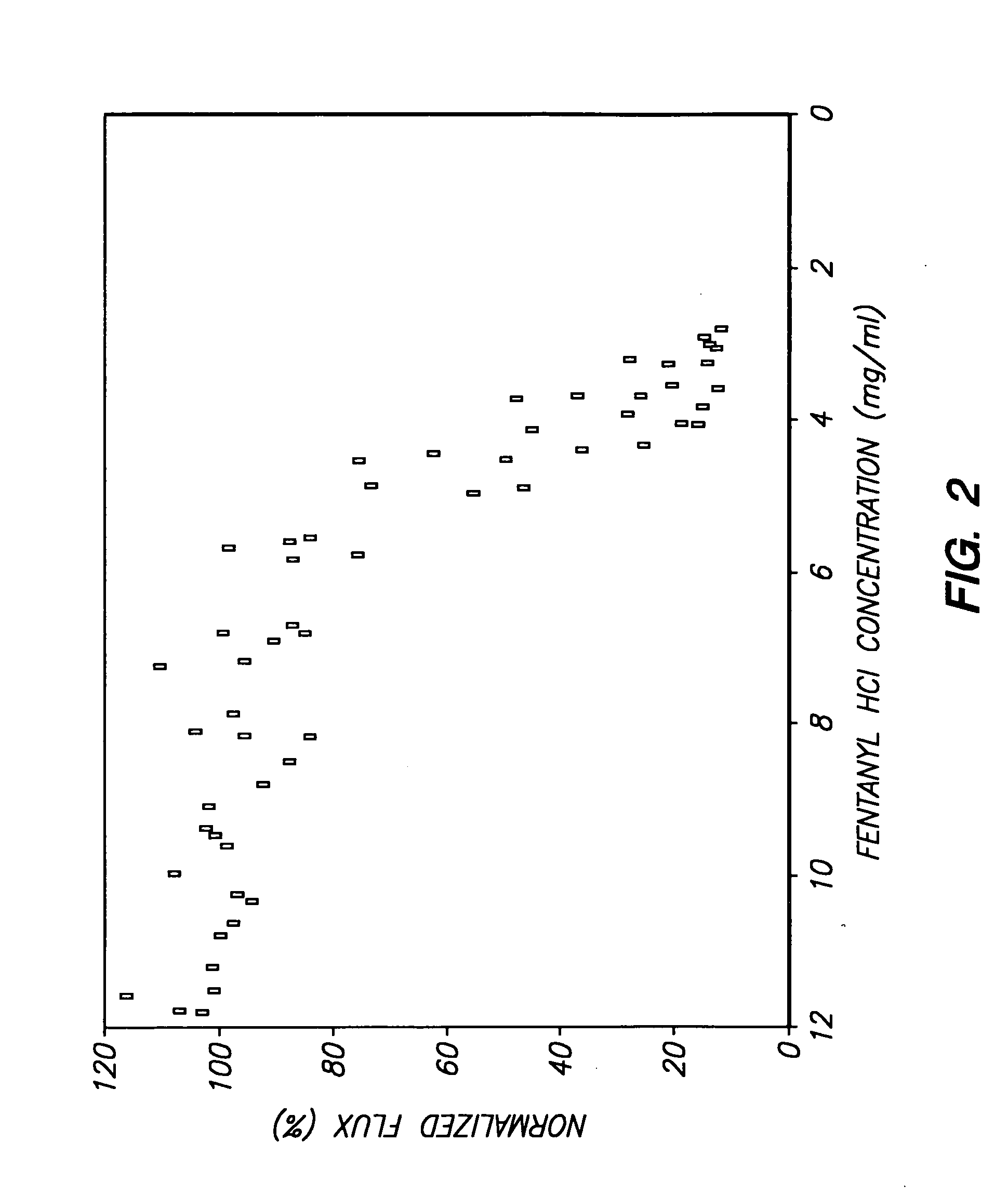
The invention provides an improved electrotransport drug delivery system for analgesic drugs, namely fentanyl and sufentanil. The fentanyl / sufentanil is provided as a water soluble salt (eg, fentanyl hydrochloride) dispersed in a hydrogel formulation for use in an electrotransport device (10). In accordance with one aspect of the invention, the concentration of fentanyl / sufentanil in the donor reservoir (26) solution is above a predetermined minimum concentration, whereby the transdermal electrotransport flux of fentanyl / sufentanil is maintained independent of the concentration of fentanyl / sufentanil in solution. In accordance with a second aspect of the present invention, the donor reservoir (26) of the electrotransport delivery device (10) is comprised of silver and the donor reservoir (26) contains a predetermined “excess” loading of fentanyl / sufentanil halide to prevent silver ion migration with attendant skin discoloration. In accordance with a third aspect of the present invention, a transdermal electrotransport delivered dose of fentanyl / sufentanil is provided which is sufficient to induce analgesia in (eg, adult) human patients suffering from moderate-to-severe pain associated with major surgical procedures.
Owner:ALZA CORP
Compositions and methods for treatment of skin discoloration
ActiveUS20060057081A1Good lookingWhitening skinCosmetic preparationsToilet preparationsHuman skinAdditive ingredient
A cosmetically acceptable product for application to human skin is disclosed. The novel compositions are particularly suited for skin lightening and for diminishing the appearance of “dark circles” under the eyes. The compositions include any of several vasoconstrictors in a carrier with optionally added skin compatible ingredients.
Owner:EVERA LAB
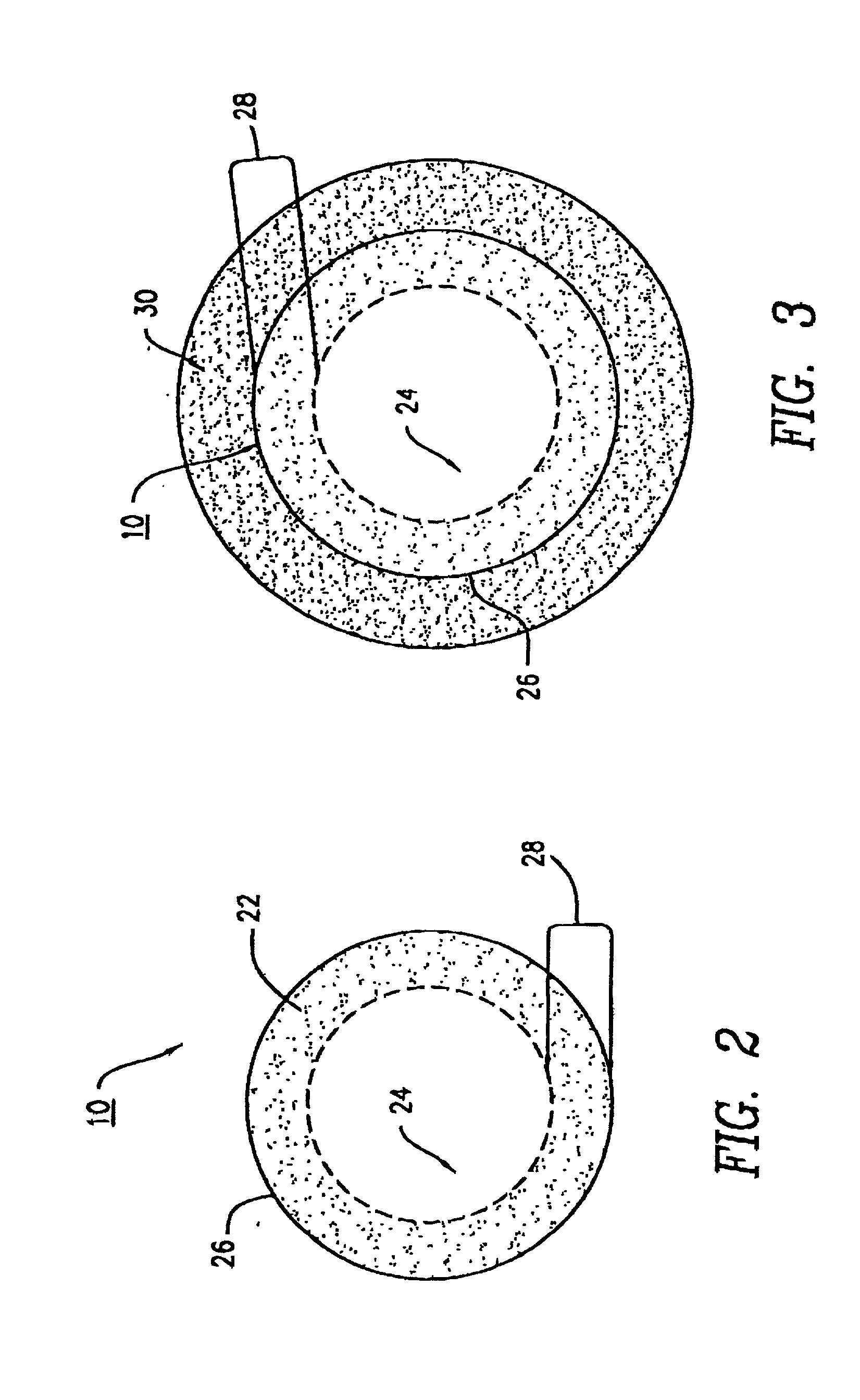
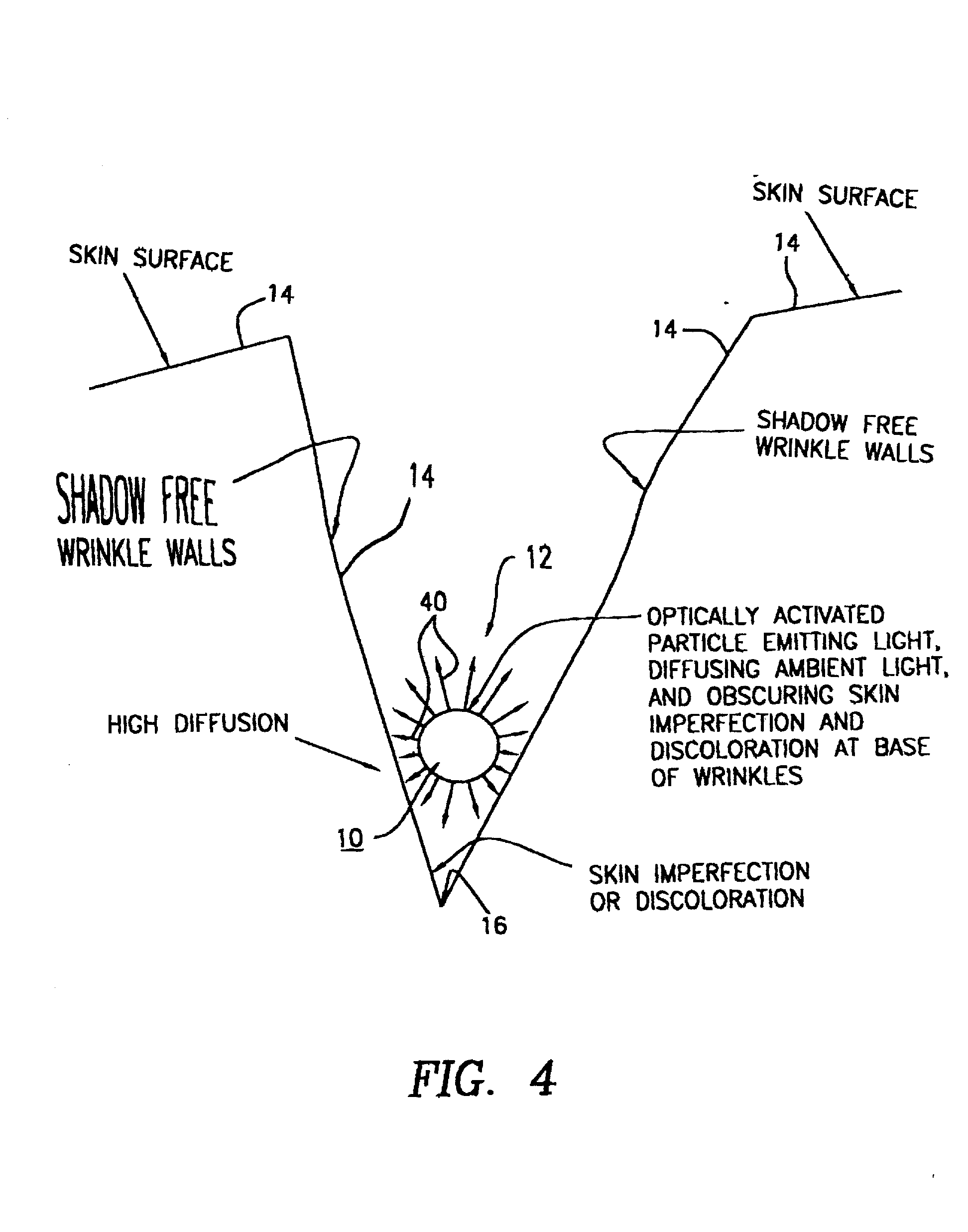
Method of using optically-activ ated particles in cosmetic preparations
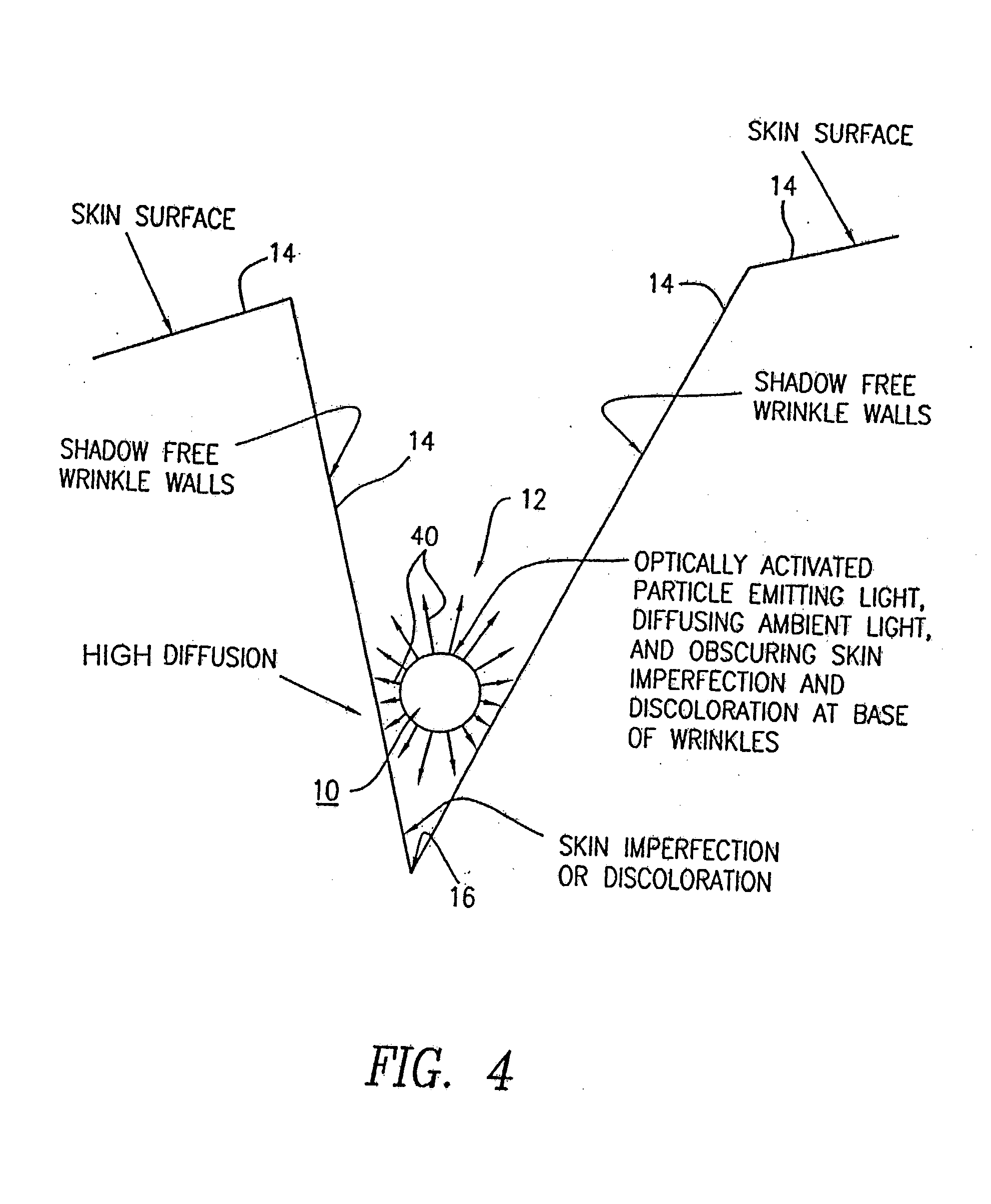
Optically-activated particles for use in cosmetic preparations. The optically-activated particles include a plurality of substrate particles selected from the group consisting of nylons, acrylics, polyesters, other plastic polymers, natural materials, regenerated cellulose, metals and minerals; an optical brightener chemically bonded to each of the plurality of substrate particles to form integral units in the form of optically-activated particles for diffusing light to reduce the visual perception of skin imperfections, including cellulite, shadows, skin discolorations, and wrinkles; and each of the optically-activated particles are encapsulated with a UV transparent coating to increase the diffusion light to further reduce the visual perception of the skin imperfections. The encapsulated optically-activated particles are able to absorb ultraviolet radiation and emit visible light; and the encapsulated optically-activated particles are able to both scatter and absorb light in a diffuse manner in order to reduce the visual perception of skin imperfections, including cellulite, wrinkles, shadows, and skin discolorations, when the optically-activated particles are applied to the skin surface. The encapsulated optically-activated particles are used in the making of cosmetic preparations such as skin lotions, creams, shampoos, body and skin rinses, bath gels, soaps, hair conditioners, color conditioners and rinses, hair color solutions, foundation powders (compressed or loose), tooth pastes, oral rinses, and any acceptable cosmetic vehicle or medium.
Owner:PNC BANK NAT ASSOC
Method and device for transdermal delivery of fentanyl and sufentanil
InactiveUS20050131337A1Improved transdermal electrotransport deliveryImprove efficiencyBiocideNervous disorderAnalgesics drugsMedicine
Owner:ALZA CORP
Cosmetic Composition
InactiveUS20100003205A1Increase coverageMinimizing reflectanceBiocideHeavy metal active ingredientsSkin appearanceMedicine
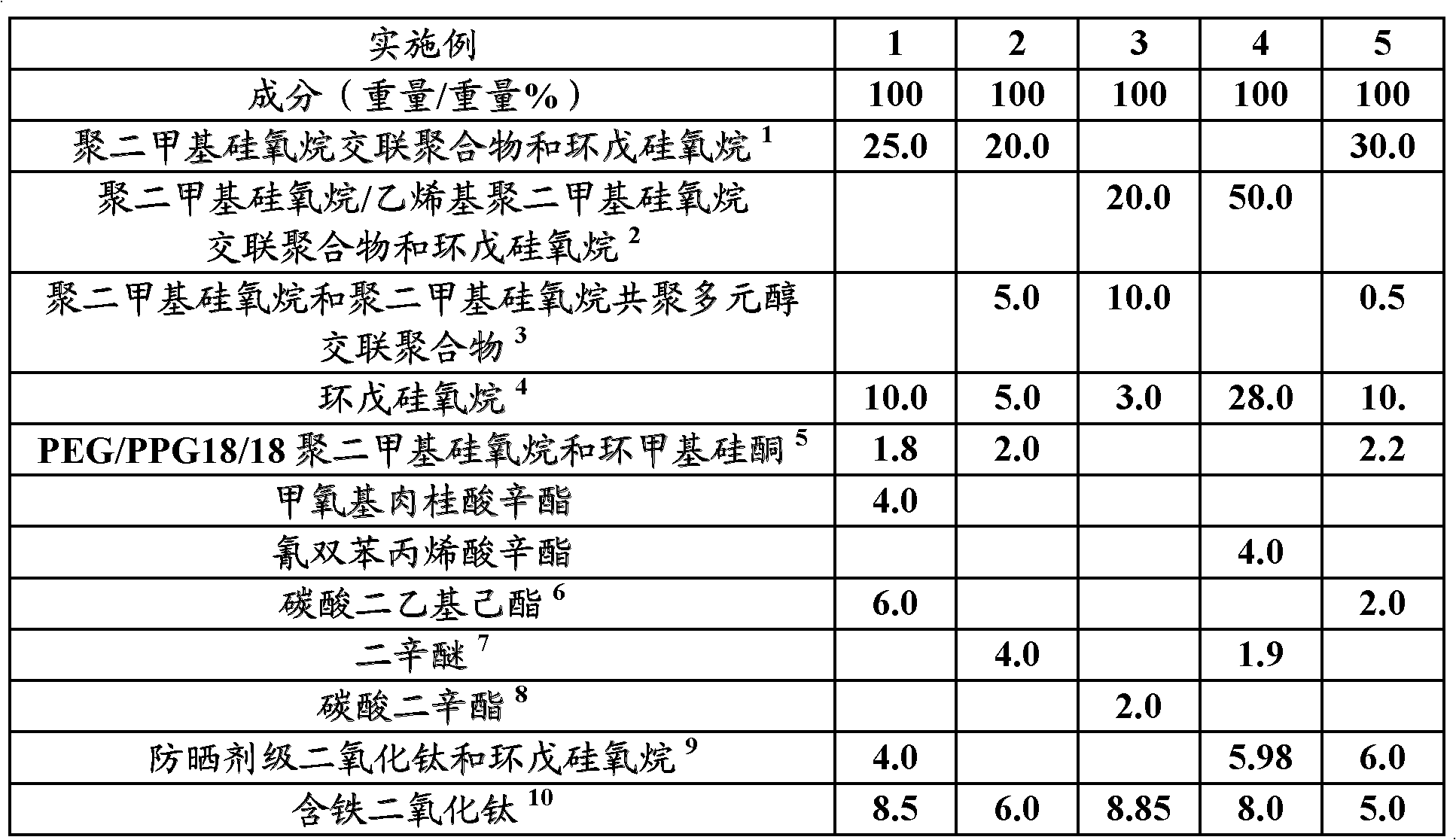
Cosmetic composition providing a high coverage of skin while retaining a natural skin appearance comprising iron oxide particles having an average primary particle size of less than or equal to 100 nm, iron-containing titanium dioxide particles having an average primary particle size of at least 105 nm and comprising from 1% to 15% iron by weight of the titanium dioxide, and a cosmetically acceptable carrier and its use as a foundation and / or as a composition to correct skin discoloration surrounding the eye.
Owner:THE PROCTER & GAMBLE COMPANY
Cosmetic Composition
InactiveUS20100074928A1Increase coverageMinimizing reflectanceHeavy metal active ingredientsCosmetic preparationsSkin appearanceTio2 particle
Cosmetic composition providing a high coverage of skin while retaining a natural skin appearance comprising iron oxide particles having an average surface area from 30 m2 / g to 150 m2 / g, iron-containing titanium dioxide particles having an average surface area from 1 m2 / g to 30 m2 / g and comprising from 1% to 15% iron by weight of the titanium dioxide, and a cosmetically acceptable carrier and its use as a foundation and / or as a composition to correct skin discoloration surrounding the eye.
Owner:NOXELL CORP
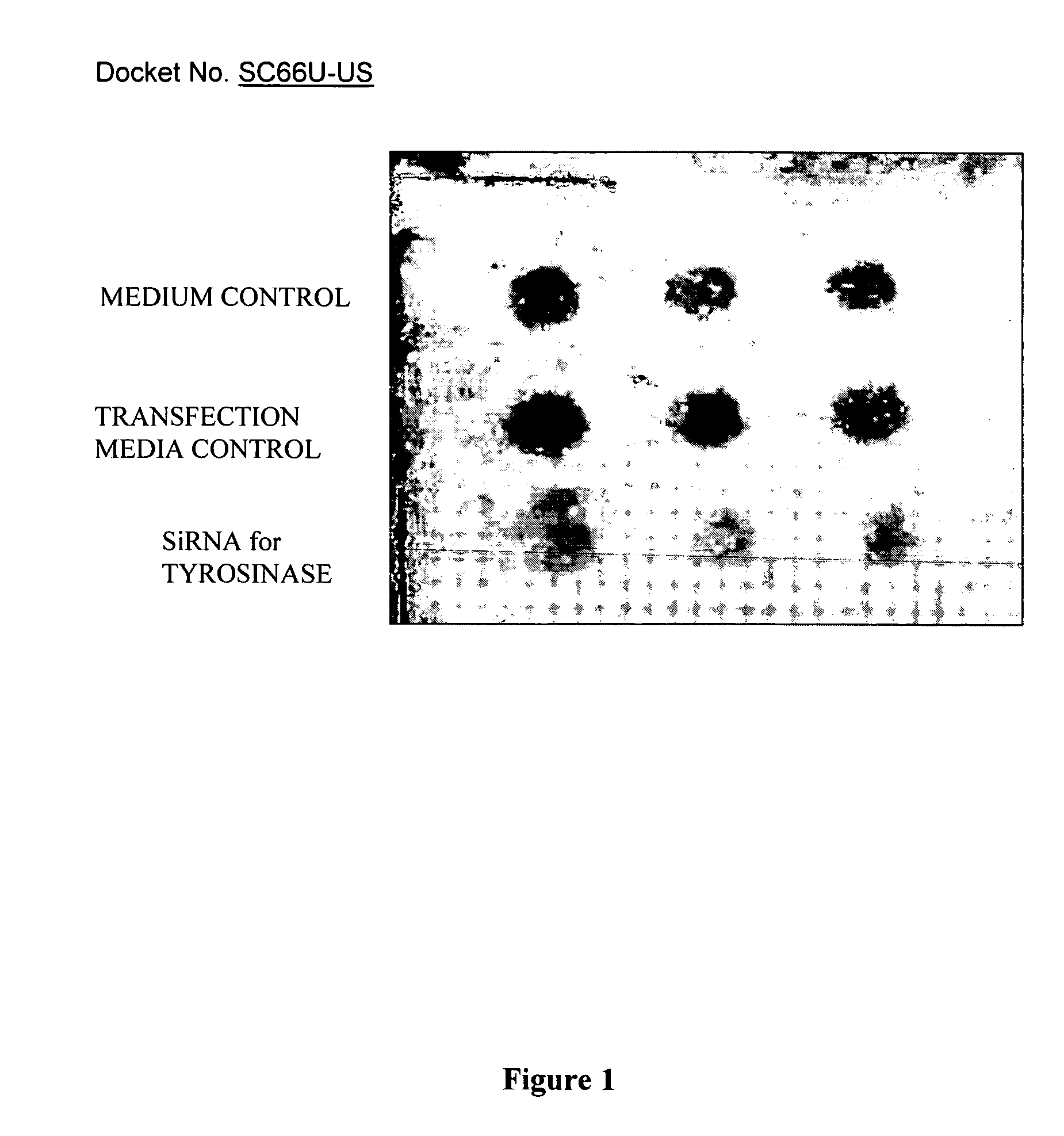
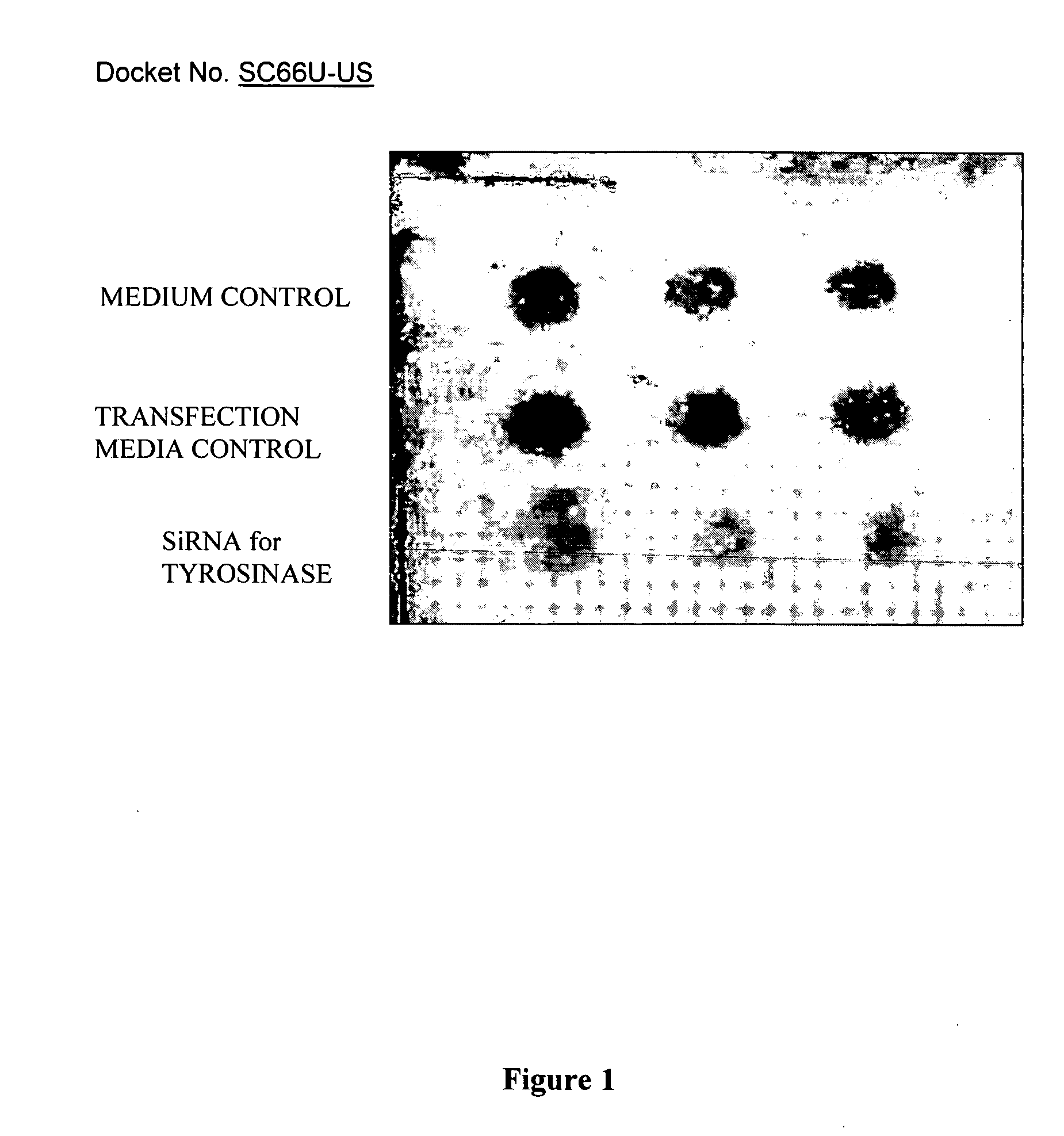
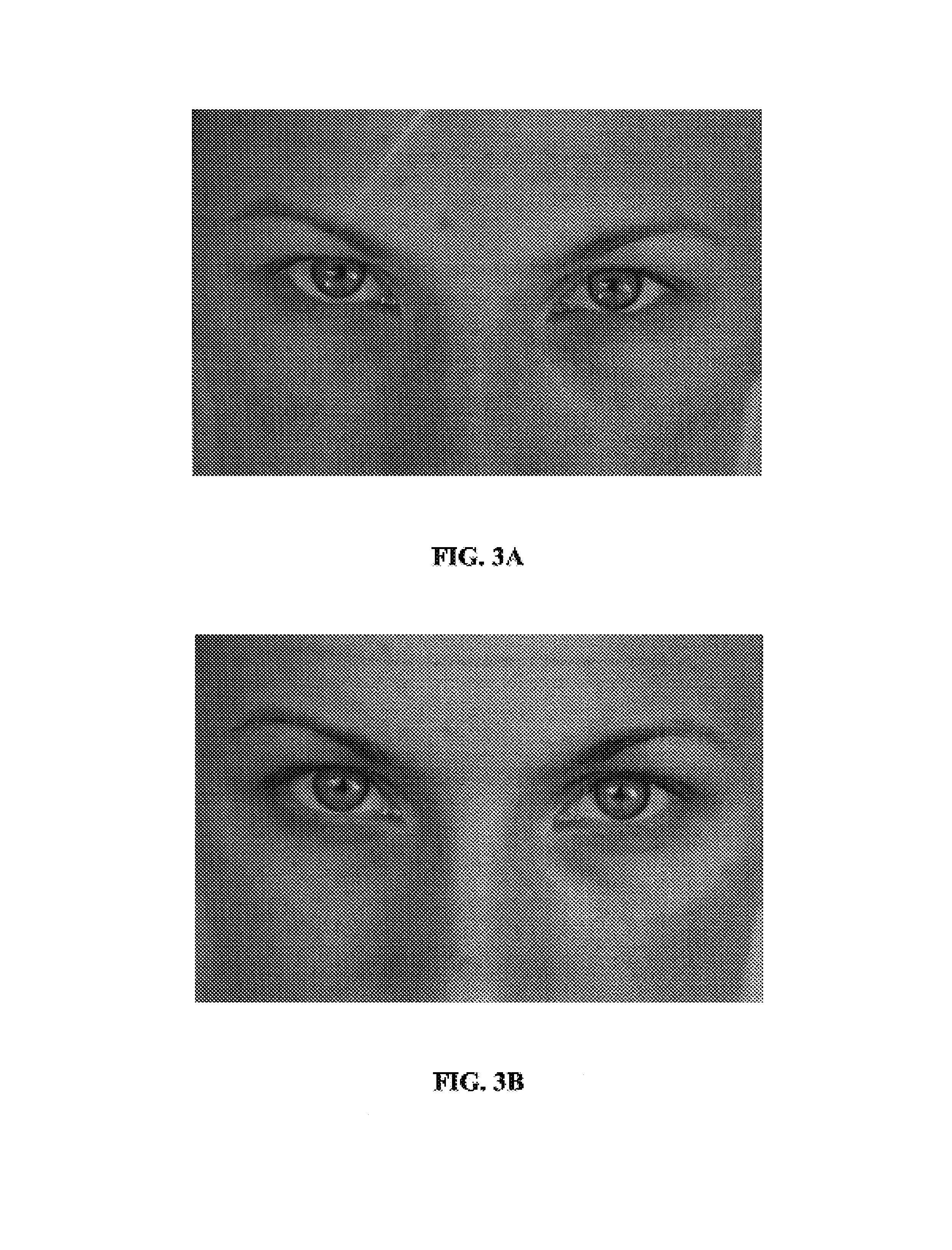
si-RNA-mediated gene silencing technology to inhibit tyrosinase and reduce pigmentation
InactiveUS7504385B2Inhibit productionImprove aestheticsBiocideSugar derivativesHypopigmentationOligomer
Owner:AVON PROD INC
Method of using optically-activated particles in cosmetic preparations
Optically-activated fixed particles for use in cosmetic, toiletries, or pharmaceutical preparations. The optically-activated fixed particles include a plurality of substrate particles and a fluorescent compound fixed to each of the plurality of substrate particles to form integral units in the form of optically-activated fixed particles for reducing the visual perception of skin imperfections, including cellulite, shadows, skin discolorations, wrinkles, and mild scars. Each of the optically-activated fixed particles are optionally encapsulated with a transparent or translucent coating. The unencapsulated and encapsulated optically-activated fixed particles are able to absorb visible light at certain wavelengths and emit visible light at longer wavelengths; and are able to both absorb and scatter light in a diffuse manner in order to reduce the visual perception of skin imperfections, including cellulite, wrinkles, shadows, skin discolorations, blotchiness, and mild scars, when the optically-activated fixed particles are applied to the skin surface.
Owner:PNC BANK NAT ASSOC
Method and Device for Transdermal Electrotransport Delivery of Fentanyl and Sufentanil
InactiveUS20090264855A1Improved transdermal electrotransport deliveryImprove efficiencyOrganic active ingredientsNervous disorderAnalgesics drugsMedicine
The invention provides an improved electrotransport drug delivery system for analgesic drugs, namely fentanyl and sufentanil. The fentanyl / sufentanil is provided as a water soluble salt (eg, fentanyl hydrochloride) dispersed in a hydrogel formulation for use in an electrotransport device (10). In accordance with one aspect of the invention, the concentration of fentanyl / sufentanil in the donor reservoir (26) solution is above a predetermined minimum concentration, whereby the transdermal electrotransport flux of fentanyl / sufentanil is maintained independent of the concentration of fentanyl / sufentanil in solution. In accordance with a second aspect of the present invention, the donor reservoir (26) of the electrotransport delivery device (10) is comprised of silver and the donor reservoir (26) contains a predetermined “excess” loading of fentanyl / sufentanil halide to prevent silver ion migration with attendant skin discoloration. In accordance with a third aspect of the present invention, a transdermal electrotransport delivered dose of fentanyl / sufentanil is provided which is sufficient to induce analgesia in (eg, adult) human patients suffering from moderate-to-severe pain associated with major surgical procedures.
Owner:ALZA CORP
si-RNA-mediated gene silencing technology to inhibit tyrosinase and reduce pigmentation
InactiveUS20050137151A1Inhibit productionImprove aestheticsBiocideSugar derivativesSkin hyperpigmentationTyrosine
The present invention describes compositions and methods for treating, preventing and improving hyperpigmentation, or other unwanted pigmentation of the skin, or other unwanted skin condition, such as age spots, aged skin, skin discoloration, etc., wherein the compositions include siRNA-gene silencing oligomers specific for tyrosinase. The compositions are used to treat a broad variety of pigmentation conditions, and are preferably applied to the skin, or are delivered by directed means to a site in need thereof.
Owner:AVON PROD INC
Method and device for transdermal electrotransport delivery of fentanyl and sufentanil
InactiveUS20050171464A1Improved transdermal electrotransport deliveryImprove efficiencyOrganic active ingredientsNervous disorderAnalgesics drugsMedicine
The invention provides an improved electrotransport drug delivery system for analgesic drugs, namely fentanyl and sufentanil. The fentanyl / sufentanil is provided as a water soluble salt (eg, fentanyl hydrochloride) dispersed in a hydrogel formulation for use in an electrotransport device (10). In accordance with one aspect of the invention, the concentration of fentanyl / sufentanil in the donor reservoir (26) solution is above a predetermined minimum concentration, whereby the transdermal electrotransport flux of fentanyl / sufentanil is maintained independent of the concentration of fentanyl / sufentanil in solution. In accordance with a second aspect of the present invention, the donor reservoir (26) of the electrotransport delivery device (10) is comprised of silver and the donor reservoir (26) contains a predetermined “excess” loading of fentanyl / sufentanil halide to prevent silver ion migration with attendant skin discoloration. In accordance with a third aspect of the present invention, a transdermal electrotransport delivered dose of fentanyl / sufentanil is provided which is sufficient to induce analgesia in (eg, adult) human patients suffering from moderate-to-severe pain associated with major surgical procedures.
Owner:ALZA CORP
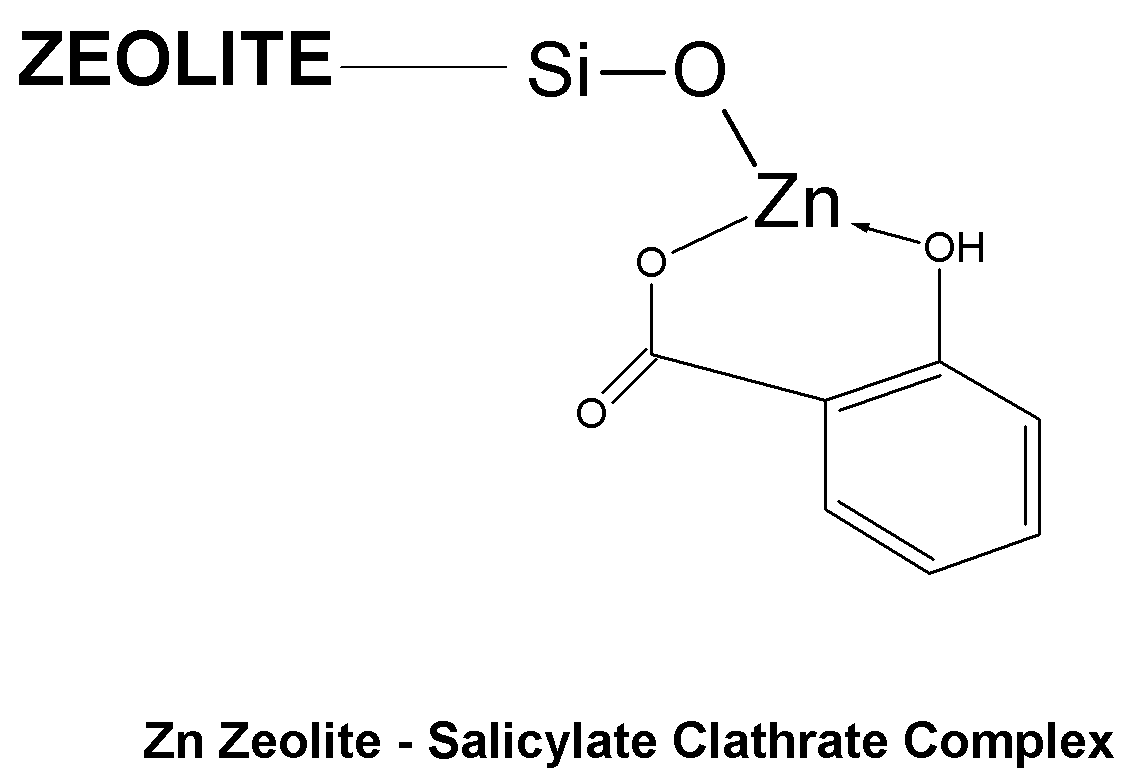
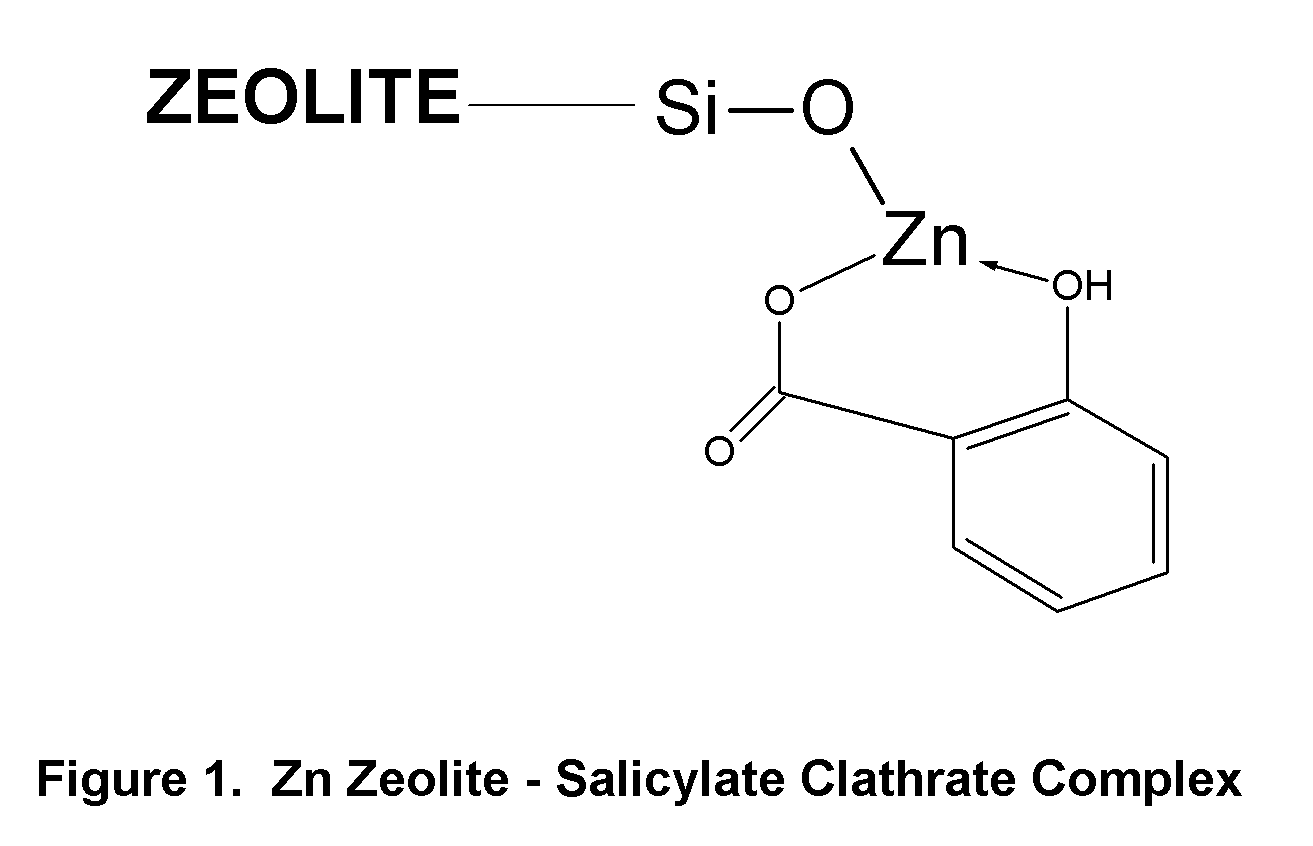
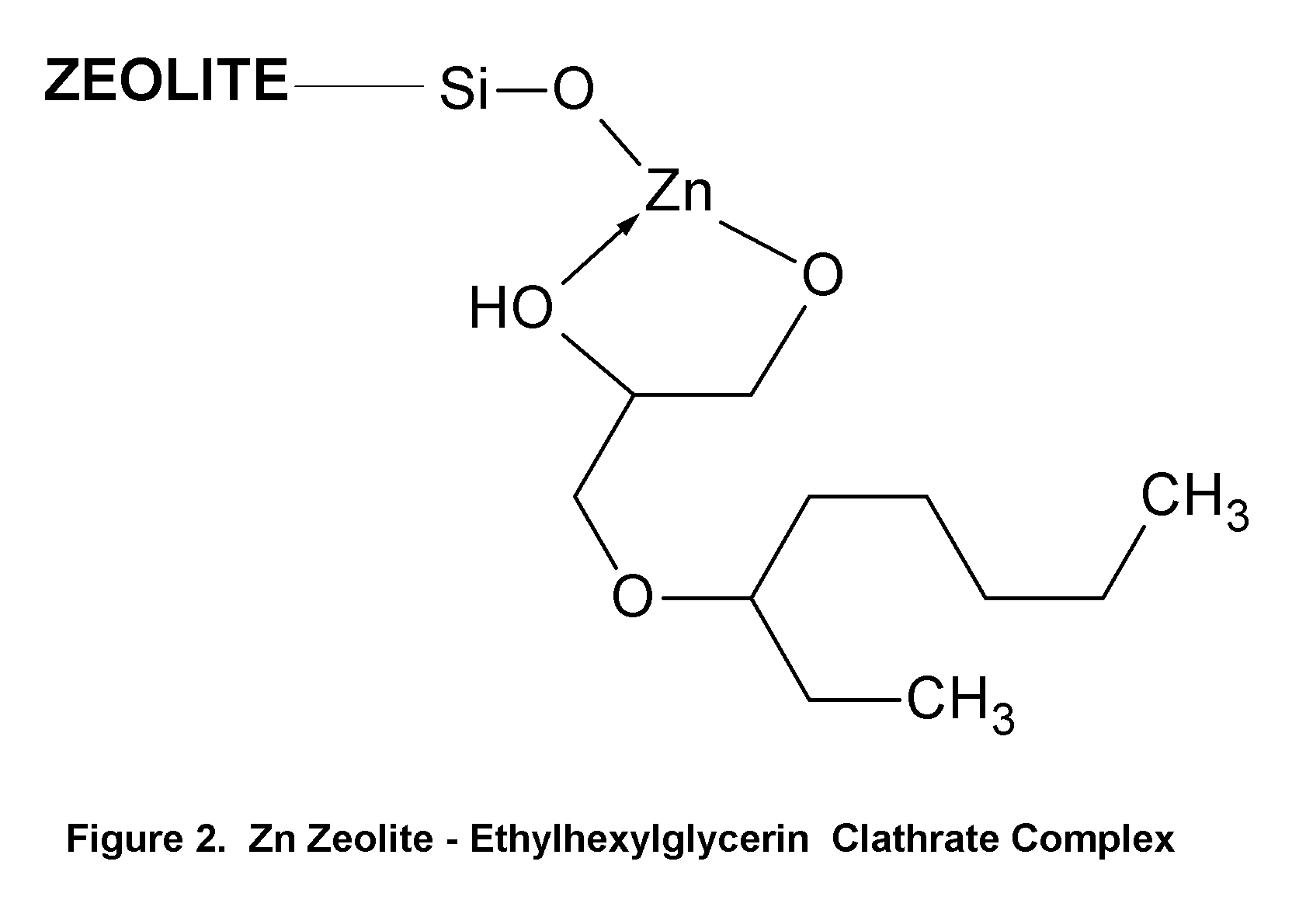
Method of Treating Skin Condition Including Acne, Skin Aging, Body Odor & Diaper Rash by Zinc Zeolite Clathrates
Owner:BIODERM RES
Enhanced protection against skin injury in humans
InactiveUS20070264222A1Reduced pHReduce skin problemsBiocideCosmetic preparationsHuman skinSkin Injury
The present invention includes compositions and methods for treating skin conditions, and more particularly, a medicinal progression of anti-oxy-vitamins to treat one or more skin conditions in a patient selected from at least one of acne, skin discoloration, comedo presence, foci of inflammation, hyper-keratinosis, enlarged sebum vessels, pores enlargement, scars or piling centers.
Owner:BIOHEALTH ADVANCE
Method of using optically-activated particles in cosmetic preparations
Optically-activated fixed particles for use in cosmetic, toiletries,-or pharmaceutical preparations. The optically-activated fixed particles include a plurality of substrate particles and a fluorescent compound fixed to each of the plurality of substrate particles to form integral units in the form of optically-activated fixed particles for reducing the visual perception of skin imperfections, including cellulite, shadows, skin discolorations, wrinkles, and mild scars. Each of the optically-activated fixed particles are optionally encapsulated with a transparent or translucent coating. The unencapsulated and encapsulated optically-activated fixed particles are able to absorb visible light at certain wavelengths and emit visible light at longer wavelengths; and are able to both absorb and scatter light in a diffuse manner in order to reduce the visual perception of skin imperfections, including cellulite, wrinkles, shadows, skin discolorations, blotchiness, and mild scars, when the optically-activated fixed particles are applied to the skin surface.
Owner:PNC BANK NAT ASSOC
Compositions and methods for treatment of skin discoloration
ActiveUS7288263B2Good lookingWhitening skinCosmetic preparationsToilet preparationsAdditive ingredientVasoconstrictor Agents
A cosmetically acceptable product for application to human skin is disclosed. The novel compositions are particularly suited for skin lightening and for diminishing the appearance of “dark circles” under the eyes. The compositions include any of several vasoconstrictors in a carrier with optionally added skin compatible ingredients.
Owner:EVERA LAB
si-RNA-Mediated Gene Silencing Technology To Inhibit Tyrosinase And Reduce Pigmentation
ActiveUS20090202458A1Inhibit productionImprove aestheticsCosmetic preparationsHair cosmeticsHypopigmentationOligomer
The present invention describes compositions and methods for treating, preventing and improving hyperpigmentation, or other unwanted pigmentation of the skin, or other unwanted skin condition, such as age spots, aged skin, skin discoloration, etc., wherein the compositions include siRNA-gene silencing oligomers specific for tyrosinase. The compositions are used to treat a broad variety of pigmentation conditions, and are preferably applied to the skin, or are delivered by directed means to a site in need thereof.
Owner:NEW AVON LLC
Artemisinin derivatives with natural amino acids, peptides, and amino sugars for the treatment of infection and topical condition in mammals
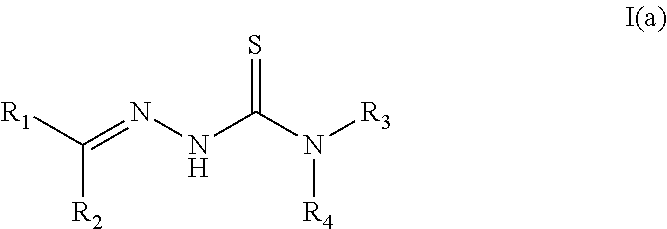
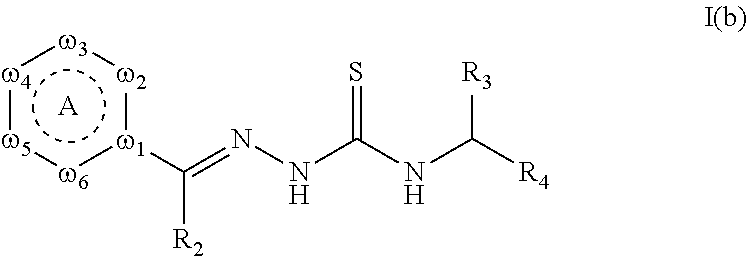
The present invention discloses certain derivatives of artemisinin and the active principles contained in Artemisia annua extracts with amino acids, peptides, and amino sugars, and isomers and salts thereof (formula I). The compounds of the present invention possess wide-spectrum antibacterial and antifungal biological activity suitable for topical or oral application for the treatment of infections and topical ailments in mammals, including acne, rosacea, topical wounds, infections, dandruff, skin disfigurements caused by infection, skin discoloration, age spots, wrinkles, excess facial oil, and veterinary problems including canine infections;
Owner:BIODERM RES
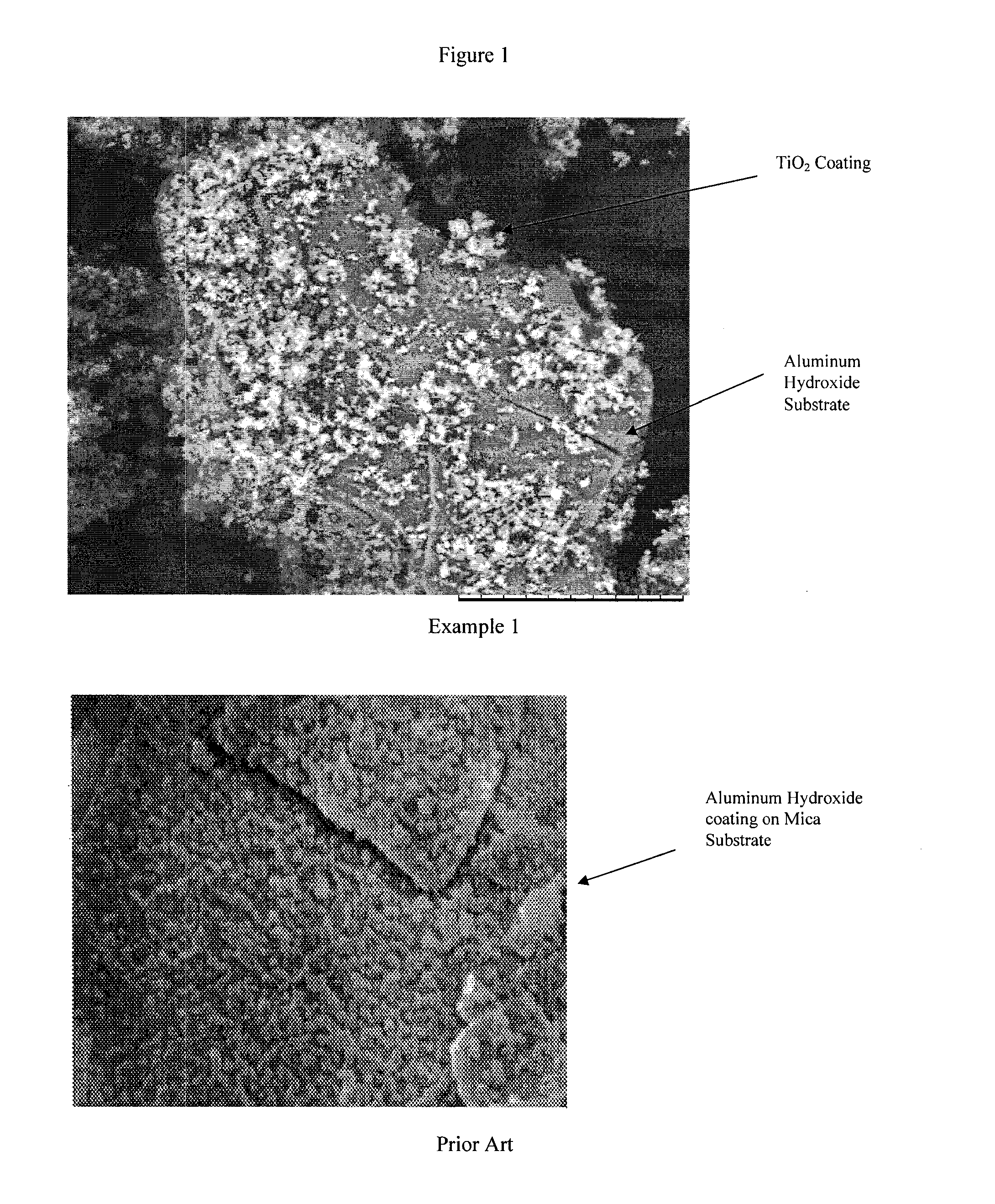
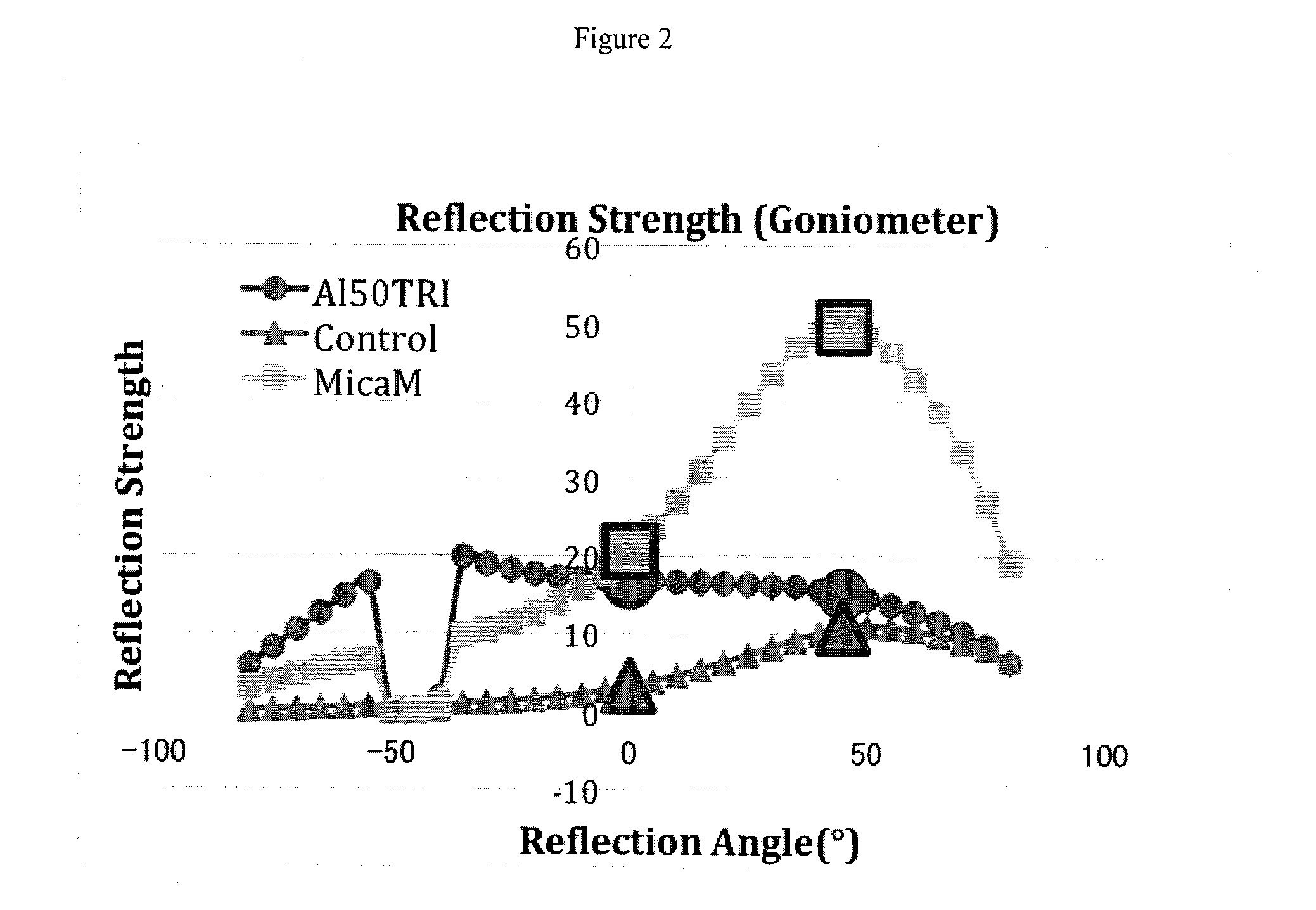
Cosmetic compositions comprising powder containing aluminum hydroxide
InactiveUS20140079745A1Increase coverageMinimizing specular reflectionCosmetic preparationsBiocideSkin appearanceMedicine
The embodiments relate to cosmetic compositions providing a high coverage to the skin while retaining a natural skin appearance. The compositions of embodiments include a powder-containing aluminum hydroxide particles having an average coated ratio of from about 60% to about 150% powder by weight of aluminum hydroxide, and a cosmetically acceptable carrier. The compositions may be useful as a foundation and / or as a composition to correct skin discoloration.
Owner:U S COSMETICS
Tyrosinase inhibitors
InactiveUS20150174034A1Signs improvedReduce pigmentationBiocideCosmetic preparationsNail stainPostinflammatory hyperpigmentation
The compositions and methods of described herein comprise novel ingredients effective to reduce unwanted pigmentation, such as skin discoloration, freckles, age spots, liver spots, sun damage, tans, pigmented acne marks, scars, pigmented birthmarks, hyperpigmentation, post-inflammatory hyperpigmentation, post-injury hyperpigmentation, melasma, cholasma, after-burn scar, nail stain, yellowing of skin, dark circles under eyes, and the like. The composition may include additional ingredients accordingly for a colored cosmetic, moisturizer, cleanser, toner, and the like.
Owner:AVON PROD INC
Artemisinin Derivatives with Natural Amino Acids, Peptides, and Amino Sugars for Skin Imperfections and Infection in Mammals
The present invention discloses certain derivatives of artemisinin and the active principles contained in Artemisia annua extracts with amino acids, peptides, and amino sugars and salts thereof (formula I). The compounds of the present invention possess wide-spectrum antibacterial and antifungal biological activity suitable for topical or oral application for the treatment of infections and topical ailments in mammals, including acne, rosacea, topical wounds, infections, dandruff, skin disfigurements caused by infection, skin discoloration, age spots, wrinkles, excess facial oil, and veterinary problems including canine infections;
Owner:BIODERM RES
Jelly-shaped longlasting makeup BB (blemish balm) cream and preparation method thereof
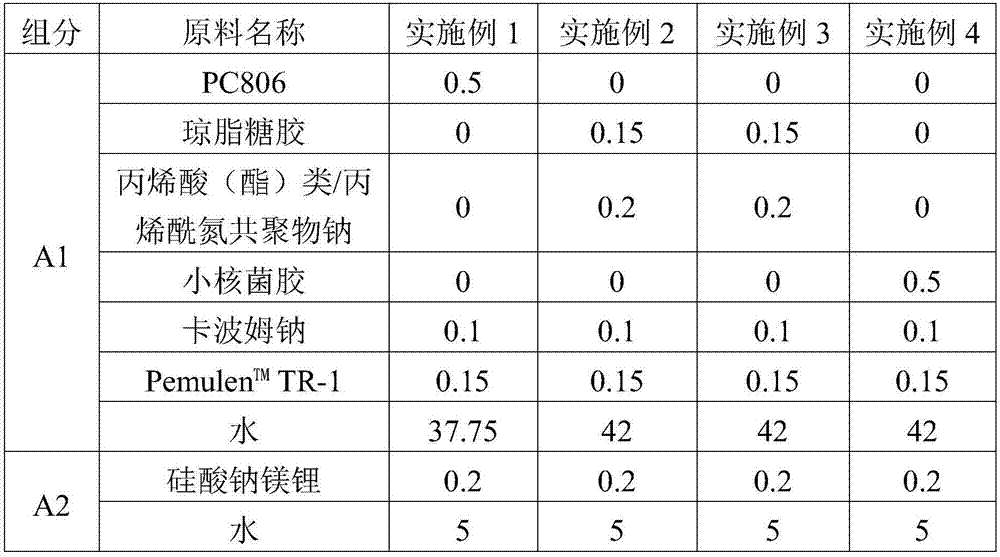
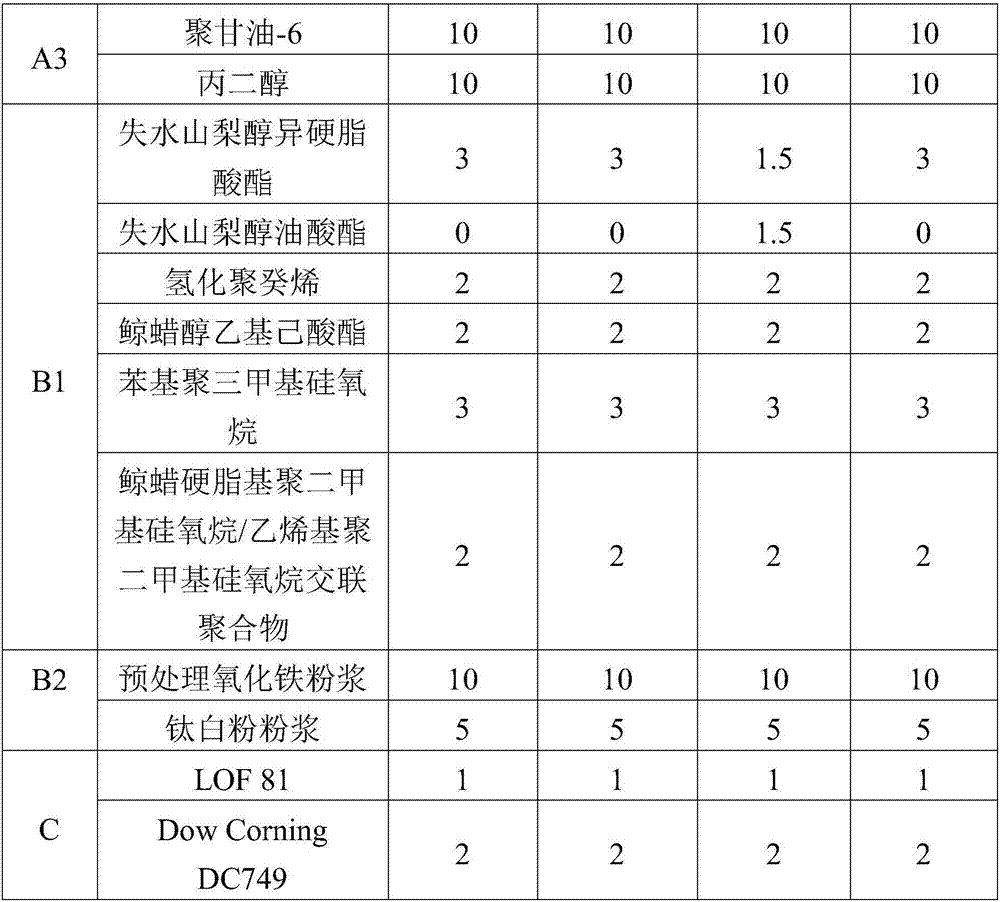
ActiveCN107970138AStrong complianceStrong concealer abilityCosmetic preparationsMake-upCross-linkAdditive ingredient
The invention provides jelly-shaped longlasting makeup BB (blemish balm) cream which is prepared from the following ingredients of lithium magnesium sodium silicate, a high-molecule rheolobic modifier, an emulsifier, pretreatment pigment powder syrup, a high-molecule film forming agent, a polyhydric alcohol wetting agent, skin moisturizing grease and deionized purified water, wherein the high-molecule rheolobic modifier is prepared from at least two in the following polymer: (A) acrylic acid (ester) / C10-30 alkanol acrylate cross-linked polymer; (B) sodium carbomer; (C) agarose gel; (D) acrylicacid (ester) / acroloyl nitrogen copolymer sodium; (E) sclerotium gum; (F) sodium alginate, cellulose and cellulose gum compound. The BB cream disclosed by the invention has the advantages of easinessin smearing, good covering effect, natural makeup, strong durability and longlasting makeup for 8 hours or more; furthermore, the skin problems of skin discoloration, acne and the like are avoided even if the BB cream is used for a long time, and the BB cream has good user evaluation.
Owner:彭氏(惠州)实业发展有限公司
Processes for reducing the appearance of pastiness or ashiness on skin
InactiveCN102076319AReduce reflectionReduce backscatterCosmetic preparationsMake-upSkin appearanceIron oxide
Disclosed are processes for reducing the appearance of ashiness or pastiness on skin by applying a cosmetic composition comprising transparent iron oxide particles thereto, iron-containing titanium dioxide particles, and a cosmetically acceptable carrier. These processes are useful to provide coverage of skin imperfections and / or skin tonal variations and / or skin discoloration surrounding the eyes on different types of skin, while, at the same time, retaining a natural skin appearance.
Owner:PROCTER & GAMBLE CO
Tyrosinase inhibitors
ActiveUS20150111897A1Signs improvedReduce pigmentationBiocideOrganic active ingredientsNail stainHyperpigmentation
The compositions and methods of described herein comprise novel ingredients effective to reduce unwanted pigmentation, such as skin discoloration, freckles, age spots, liver spots, sun damage, tans, pigmented acne marks, scars, pigmented birthmarks, hyperpigmentation, post-inflammatory hyperpigmentation, post-injury hyperpigmentation, melasma, cholasma, after-burn scar, nail stain, yellowing of skin, dark circles under eyes, and the like. The composition may include additional ingredients accordingly for a colored cosmetic, moisturizer, cleanser, toner, and the like.
Owner:NEW AVON LLC
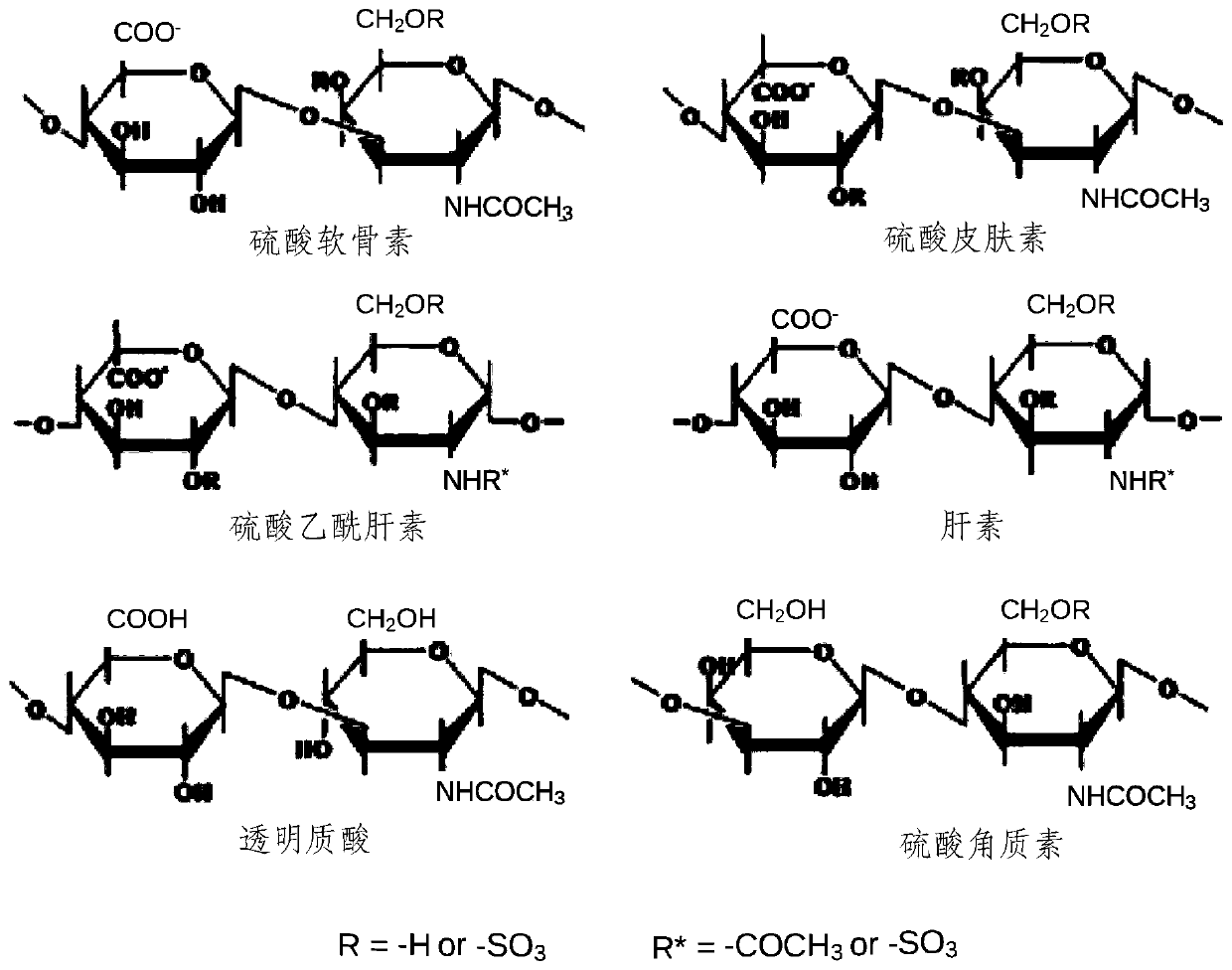
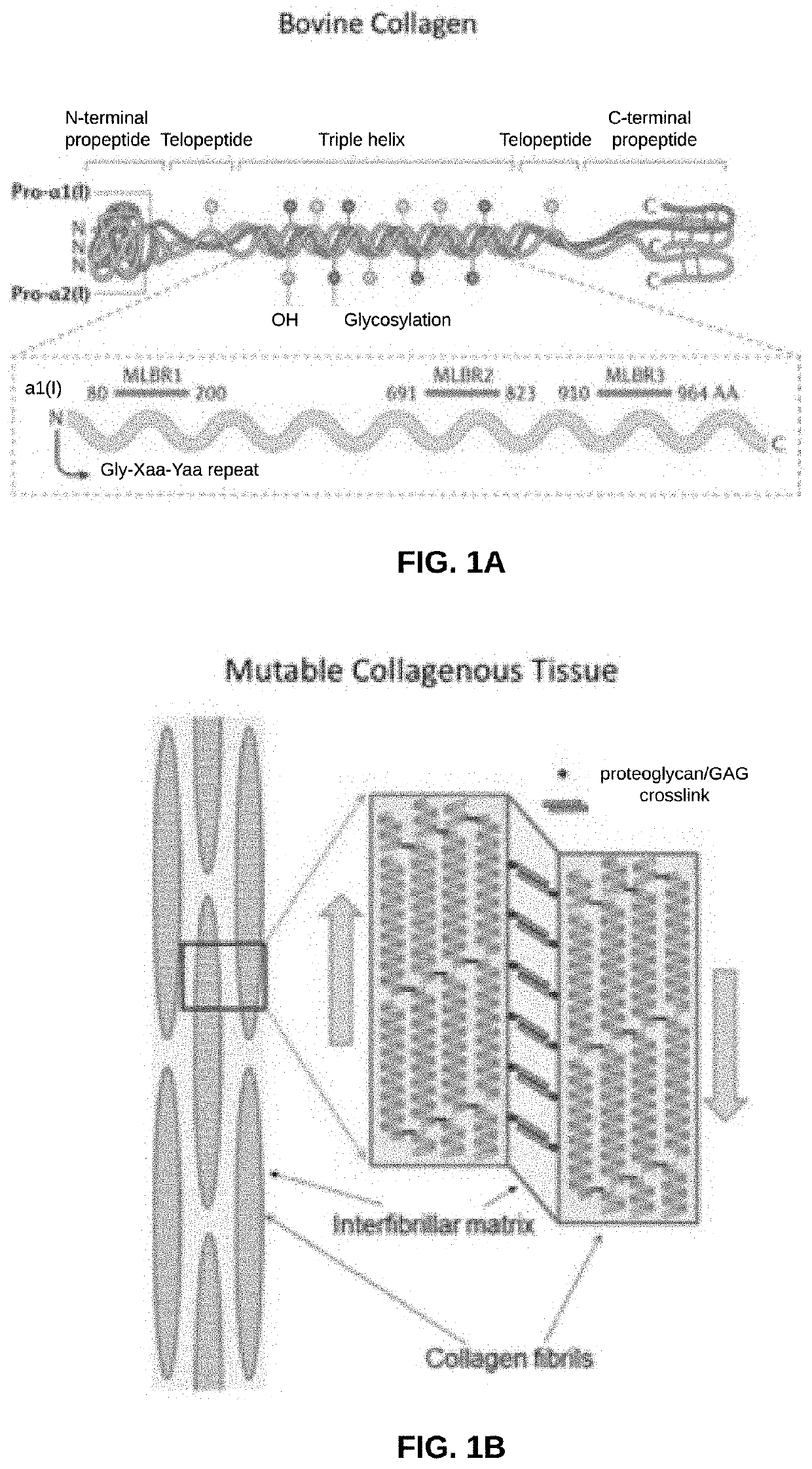
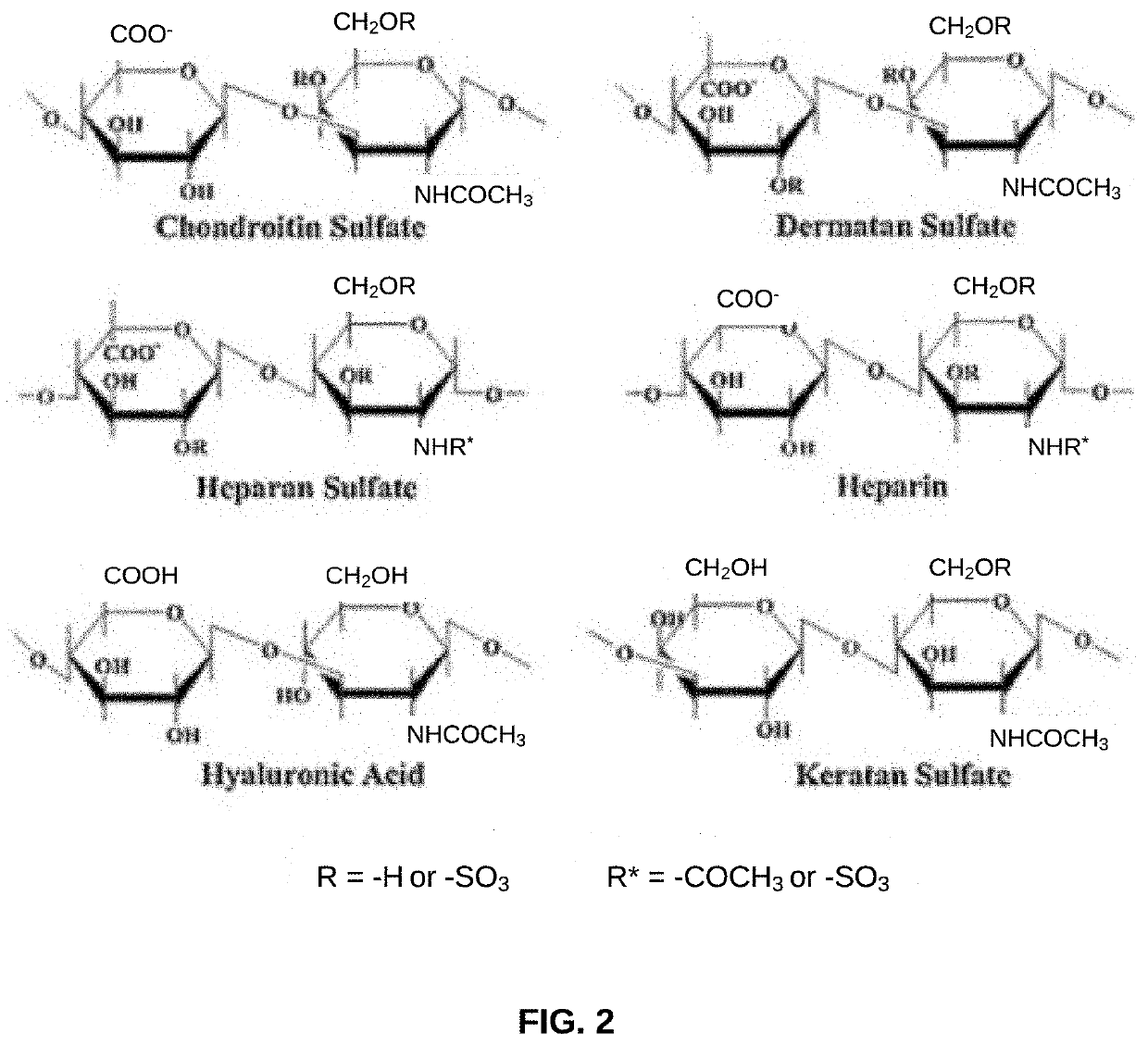
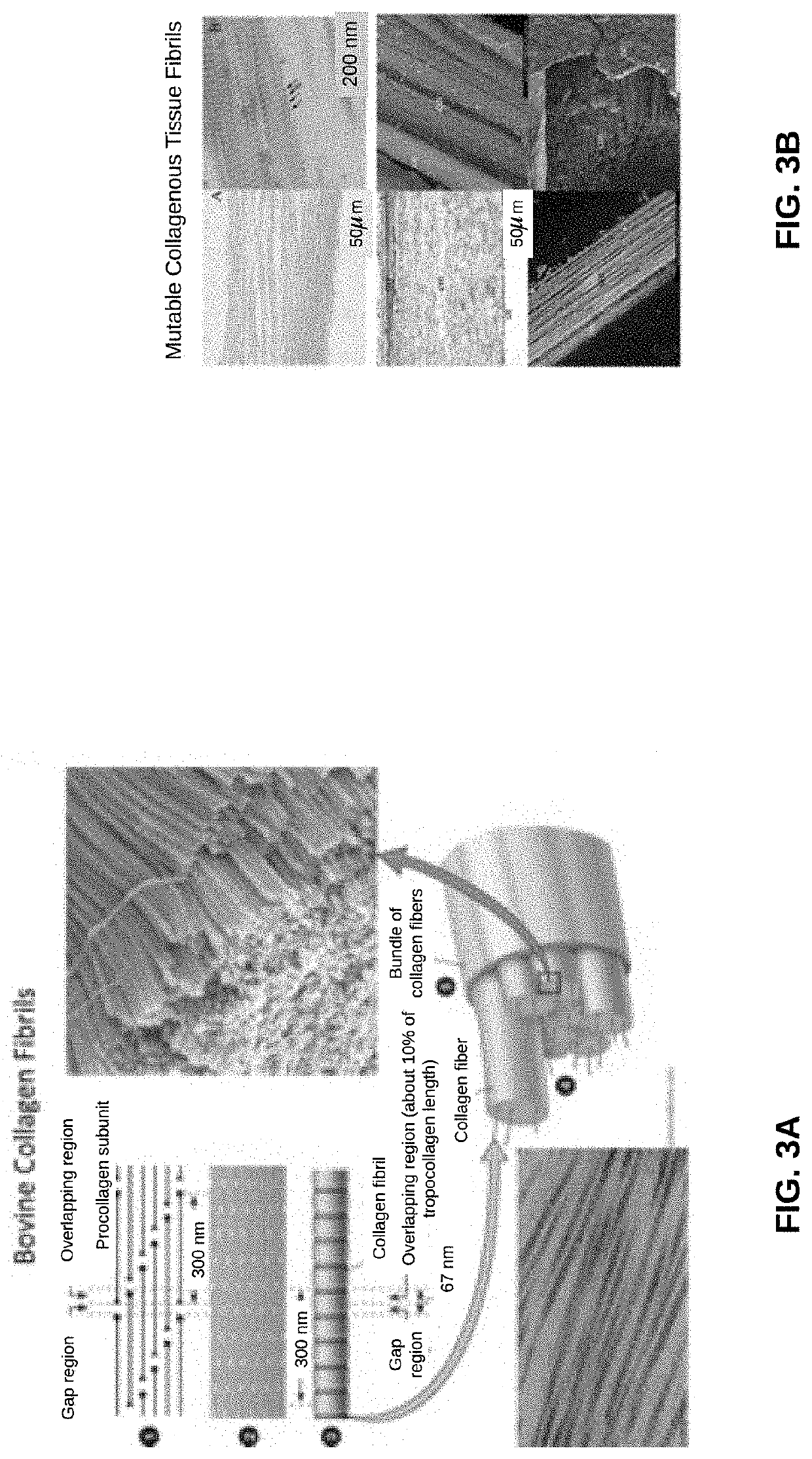
Biomaterial Devices and Topical Compositions for Treatment of Skin Abnormalities
PendingCN110624103APromote formationPromotes scar healingCosmetic preparationsOrganic active ingredientsWound dressingElectrospun nanofibers
Devices for guided tissue regeneration (GTR) include a matrix of chitosan and mutable collagenous tissue (MCT) wherein the chitosan is electrostatically bonded to the MCT to form MCT-chitosan composite material. The MCT can be isolated from invertebrate marine organisms, such as sponges, jellyfish, mollusks and echinoderms. The MCT-chitosan composite material can be formulated as a biofilm, a 3D-sponge, a hydrogel, or as an electrospun nanofiber, or the MCT-chitosan composite material can coat a biomaterial surface. The devices can include wound dressings and tissue sponges, including 3D sponges. Applications include tissue engineering and wound healing, as well as burns and other related guided tissue regeneration applications. MCT and MCT-chitosan composite material, contained in a pharmaceutically acceptable topical carrier, also has cosmeceutical applications, for treating scars, as well as skin discoloration and various pigmentation issues, including melasma / chloasma.
Owner:MARINE ESSENCE BIOSCI CORP OF USA
Biomaterial Devices and Topical Compositions for Guided Tissue Regeneration
ActiveUS20190388586A1Improve mechanical propertiesImprove efficiencyOrganic active ingredientsCosmetic preparationsElectrospun nanofiberWound dressing
Devices for guided tissue regeneration (GTR) include a matrix of chitosan and mutable collagenous tissue (MCT) wherein the chitosan is electrostatically bonded to the MCT to form MCT-chitosan composite material. The MCT can be isolated from invertebrate marine organisms, such as sponges, jellyfish, mollusks and echinoderms. The MCT-chitosan composite material can be formulated as a biofilm, a 3D-sponge, a hydrogel, or as an electrospun nanofiber, or the MCT-chitosan composite material can coat a biomaterial surface. The devices can include wound dressings and tissue sponges, including 3D sponges. Applications include tissue engineering and wound healing, as well as burns and other related guided tissue regeneration applications. MCT and MCT-chitosan composite material, contained in a pharmaceutically acceptable topical carrier, also has cosmeceutical applications, for treating scars, as well as skin discoloration and various pigmentation issues, including melasma / chloasma.
Owner:MARINE ESSENCE BIOSCI CORP OF USA
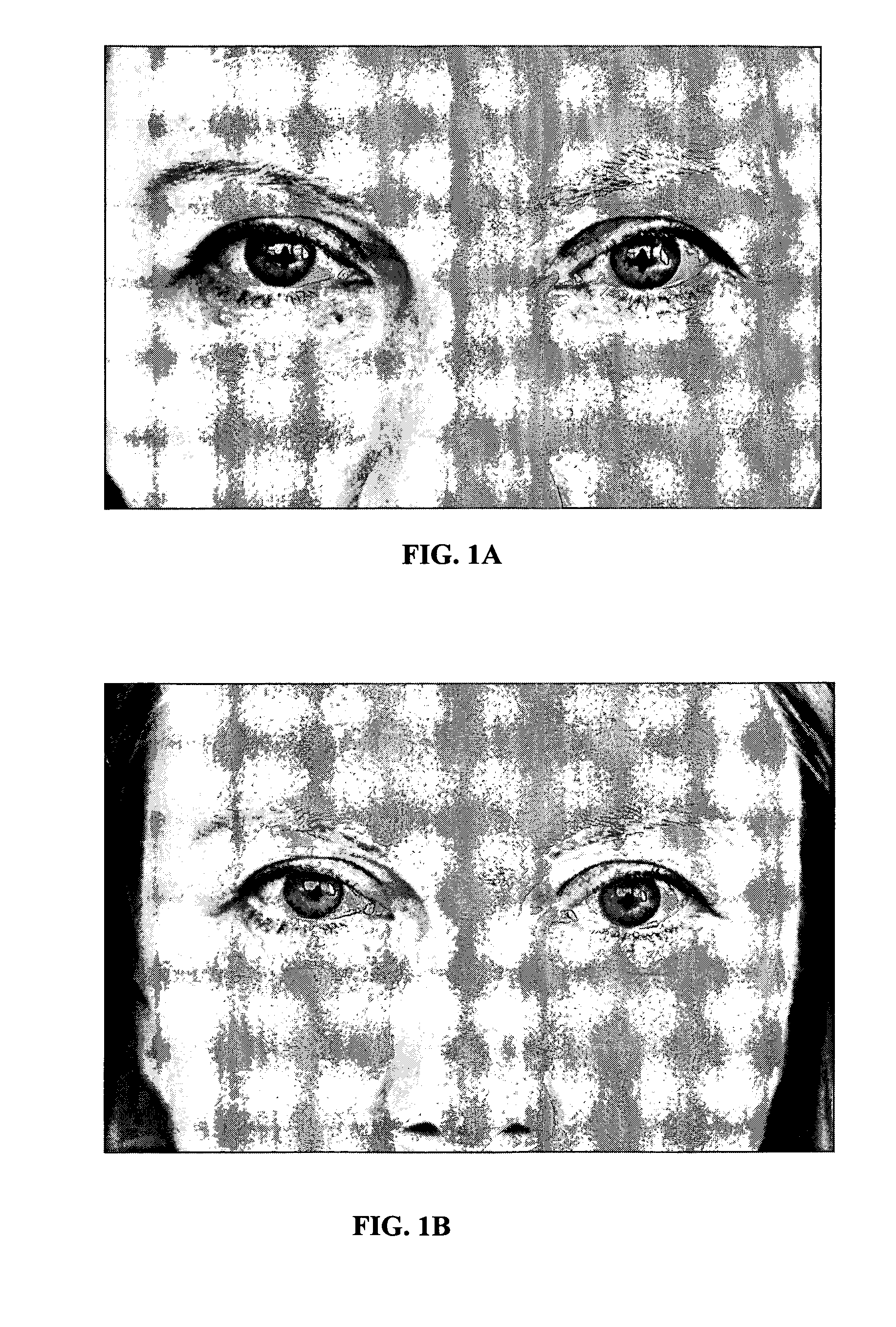
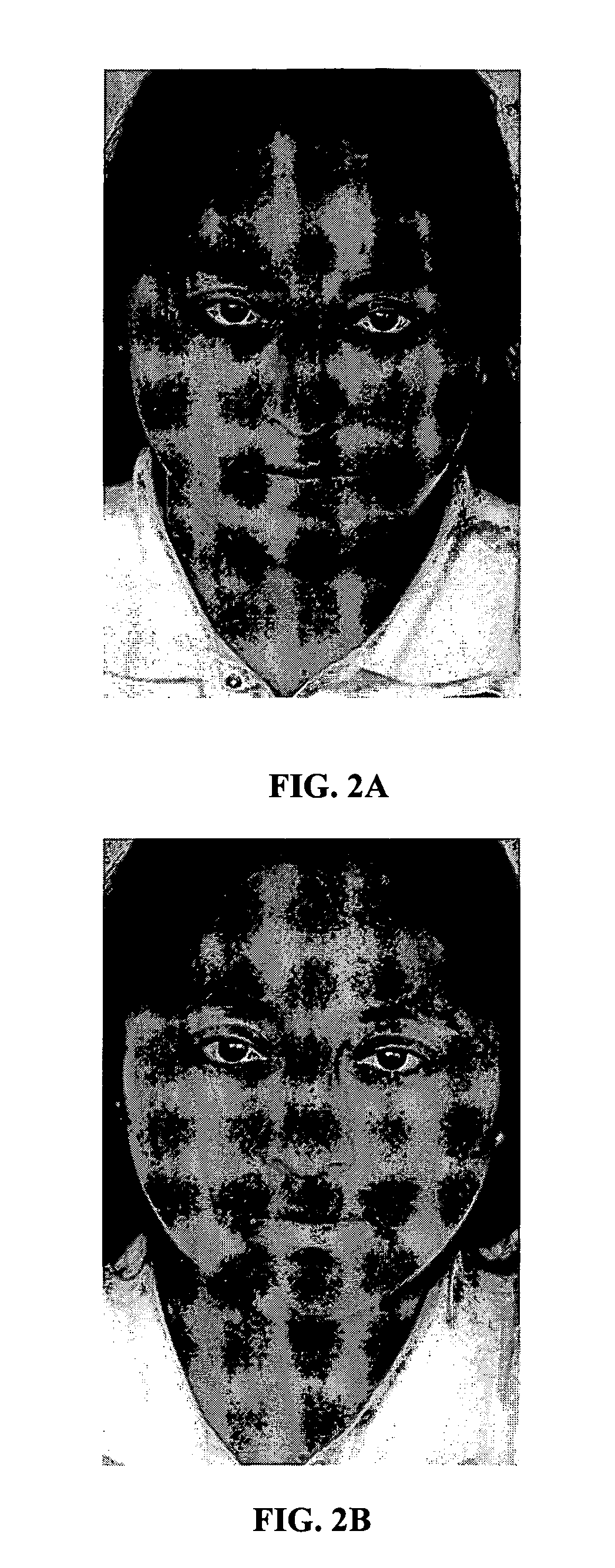
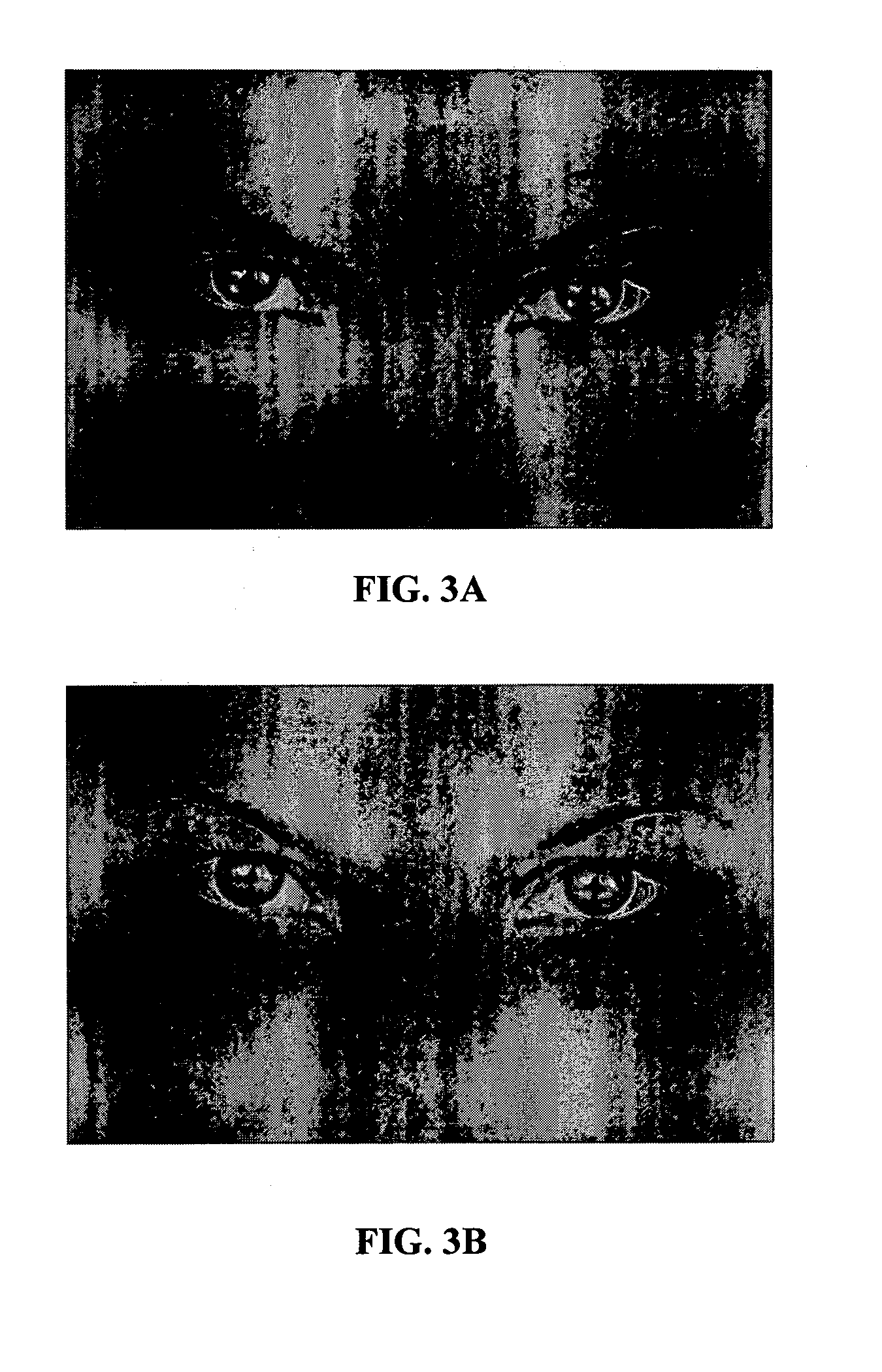
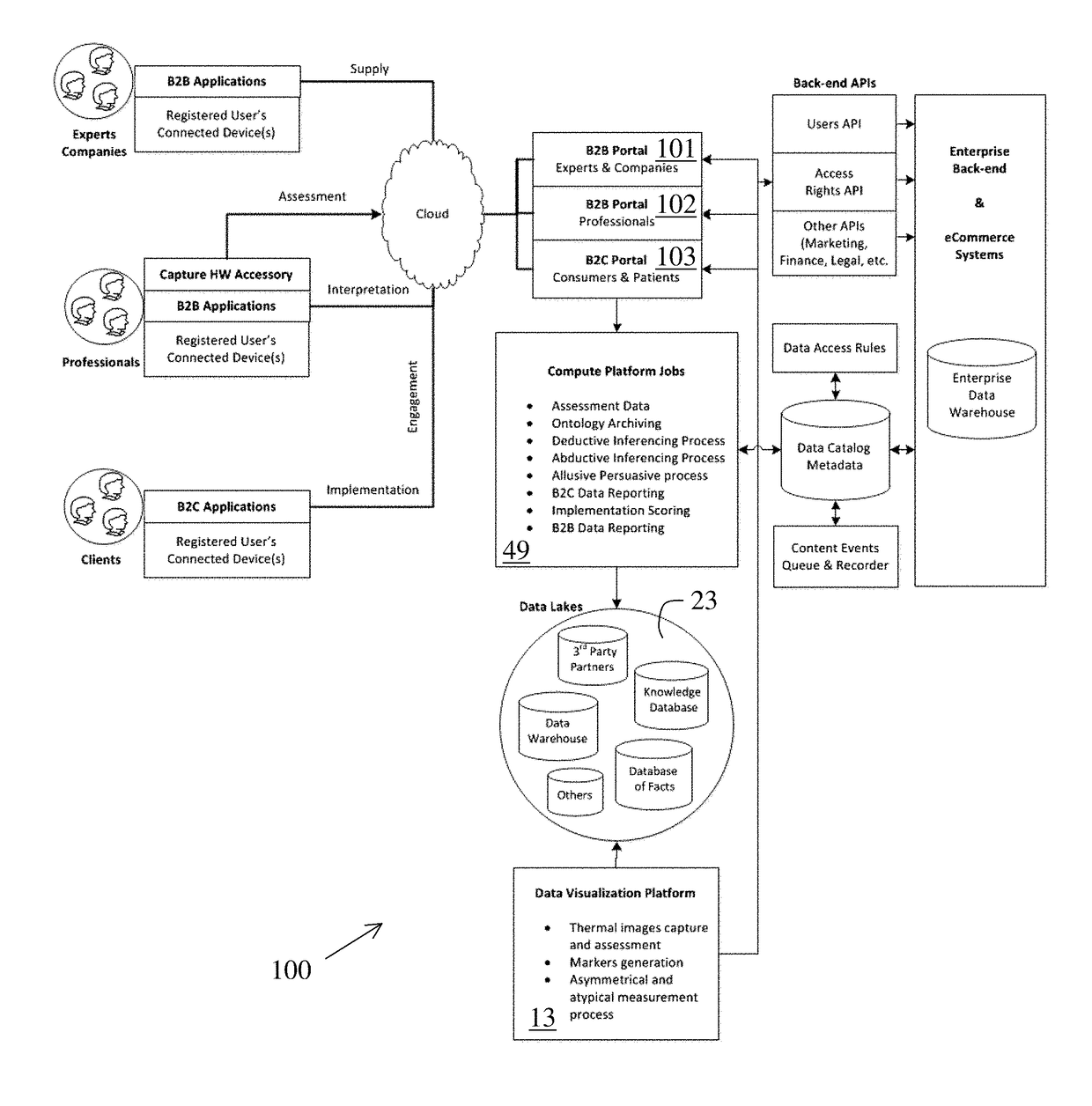
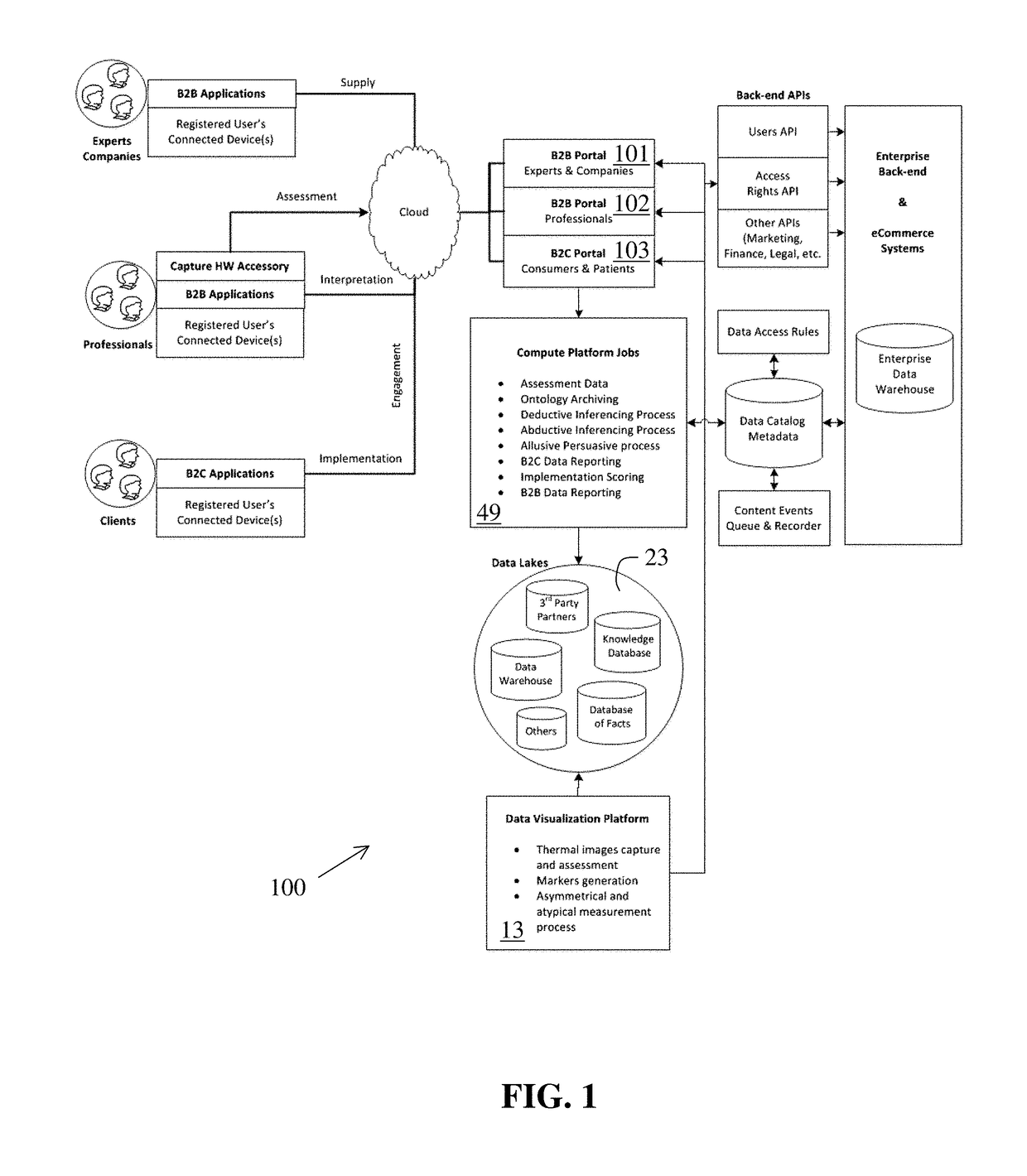
Perpetual bioinformatics and virtual colorimeter expert system
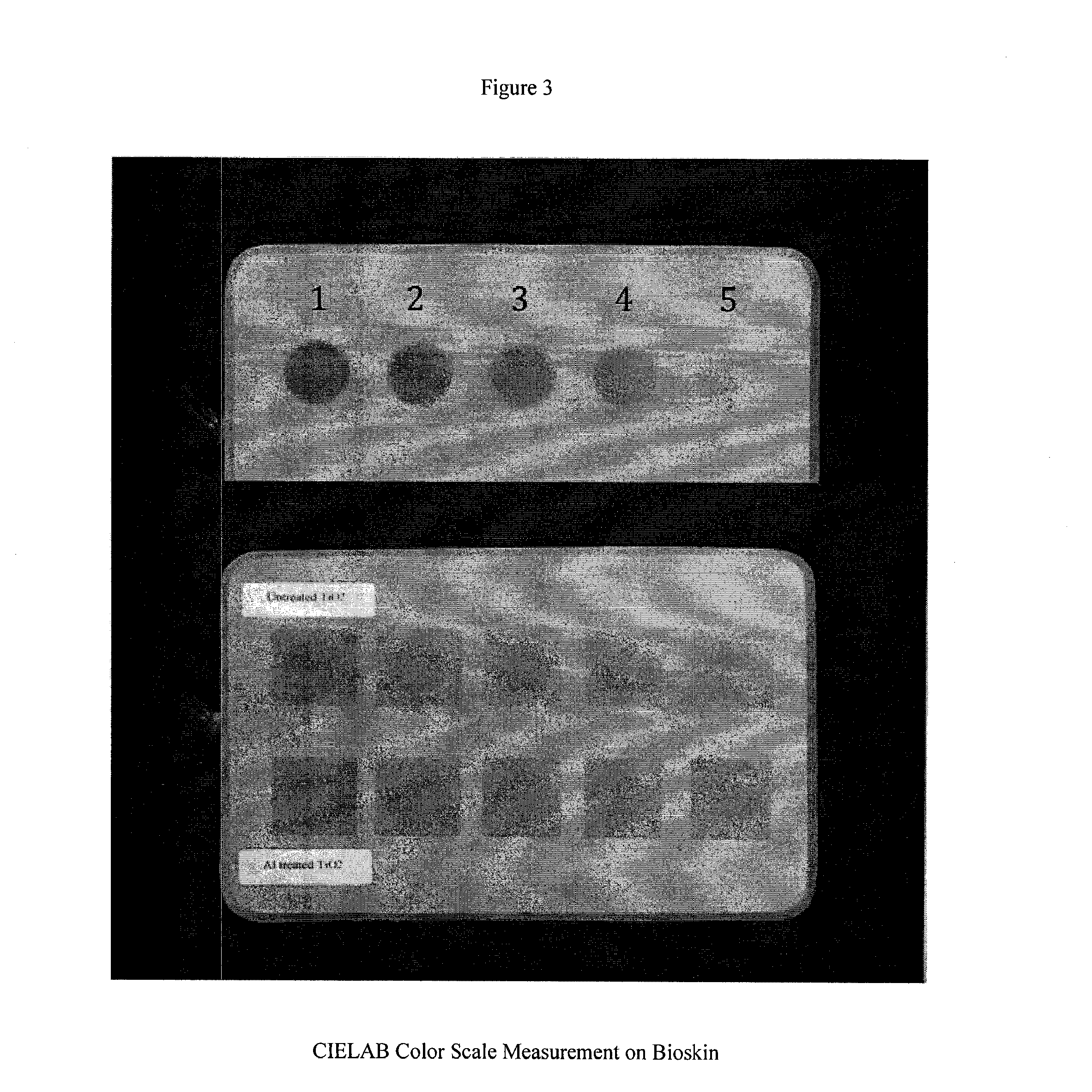
A perpetual bioinformatics and virtual colorimeter expert system platform is disclosed to remotely measure dermal and epidermal properties with precision and to enhance decision-making by offering expert diagnostic and predictive services. Some embodiments of the system, which map atypical skin discoloration may include capture means for capturing dermal images of the skin area of a user to be evaluated; control means for identifying a control tone of a user based on conditional parameters; classification means for classifying the discoloration tones from captured dermal images; measurement means for measuring discoloration tones outside the spectrum of standardized normal tones; and quantification means for calculating the severity of tone discoloration when compared to a control tone. In some embodiments the capture means may include a smartphone camera, and a virtual tristimulus colorimeter application.
Owner:ZEYIX LLC
Whitening and moisturizing body lotion and preparation method thereof
InactiveCN106551894AEasy to synthesizeAvoid lostCosmetic preparationsToilet preparationsSodium lactateDenatured alcohol
The invention relates to whitening and moisturizing body lotion and a preparation method thereof. The whitening and moisturizing body lotion is composed of, by weight, 3-5 parts of shea butter, 2-3 parts of glycerinum, 6-8 parts of silk extracts, 6-8 parts of grape seed extracts, 6-8 parts of hydrolyzed lupine protein, 2-3 parts of sodium lactate, 2-3 parts of lecithin, 3-5 parts of butanediol, 3-5 parts of propylene glycol, 2-3 parts of triethanolamine, 3-5 parts of panthenol, 2-3 parts of EDTA disodium, 3-5 parts of lactococcus ferment, 1-2 parts of denatured alcohol, 0.1-0.2 part of potassium sorbate, 0.2-0.3 part of ethanediol, 0.3-0.5 part of phenoxyethanol, 1-2 parts of ethyoxyl diethylene glycol, 0.3-0.5 part of methylparaben and 50-60 parts of water. The whitening and moisturizing body lotion has the beneficial effects of repairing injured cells, moisturizing and nourishing the skin in a long-lasting mode, avoiding skin discoloration and the color of dark skin, and making skin to be smooth and bright white.
Owner:WUXI WEITONG TRADEMARK AGENT SERVICE CO LTD
Cosmetic composition
Cosmetic composition providing a high coverage of skin while retaining a natural skin appearance comprising iron oxide particles having an average primary particle size of less than or equal to 100 nm, iron-containing titanium dioxide particles having an average primary particle size of at least 105nm and comprising from 1% to 15% iron by weight of the titanium dioxide, and a cosmetically acceptable carrier and its use as a foundation and / or as a composition to correct skin discoloration surrounding the eye.
Owner:THE PROCTER & GAMBLE COMPANY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com