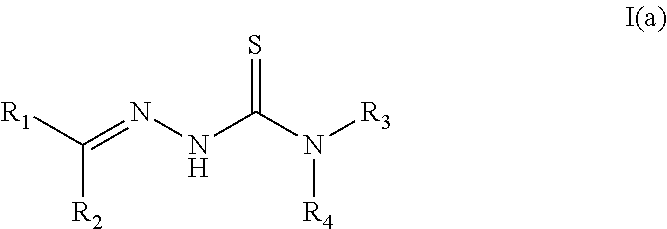
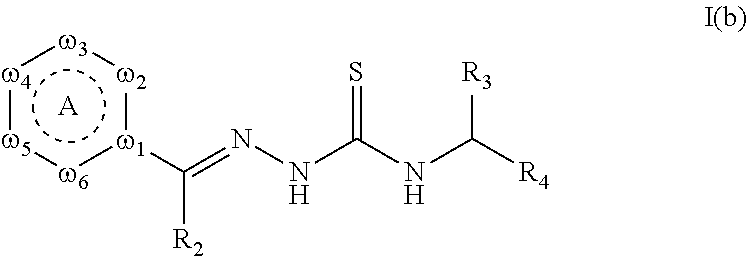
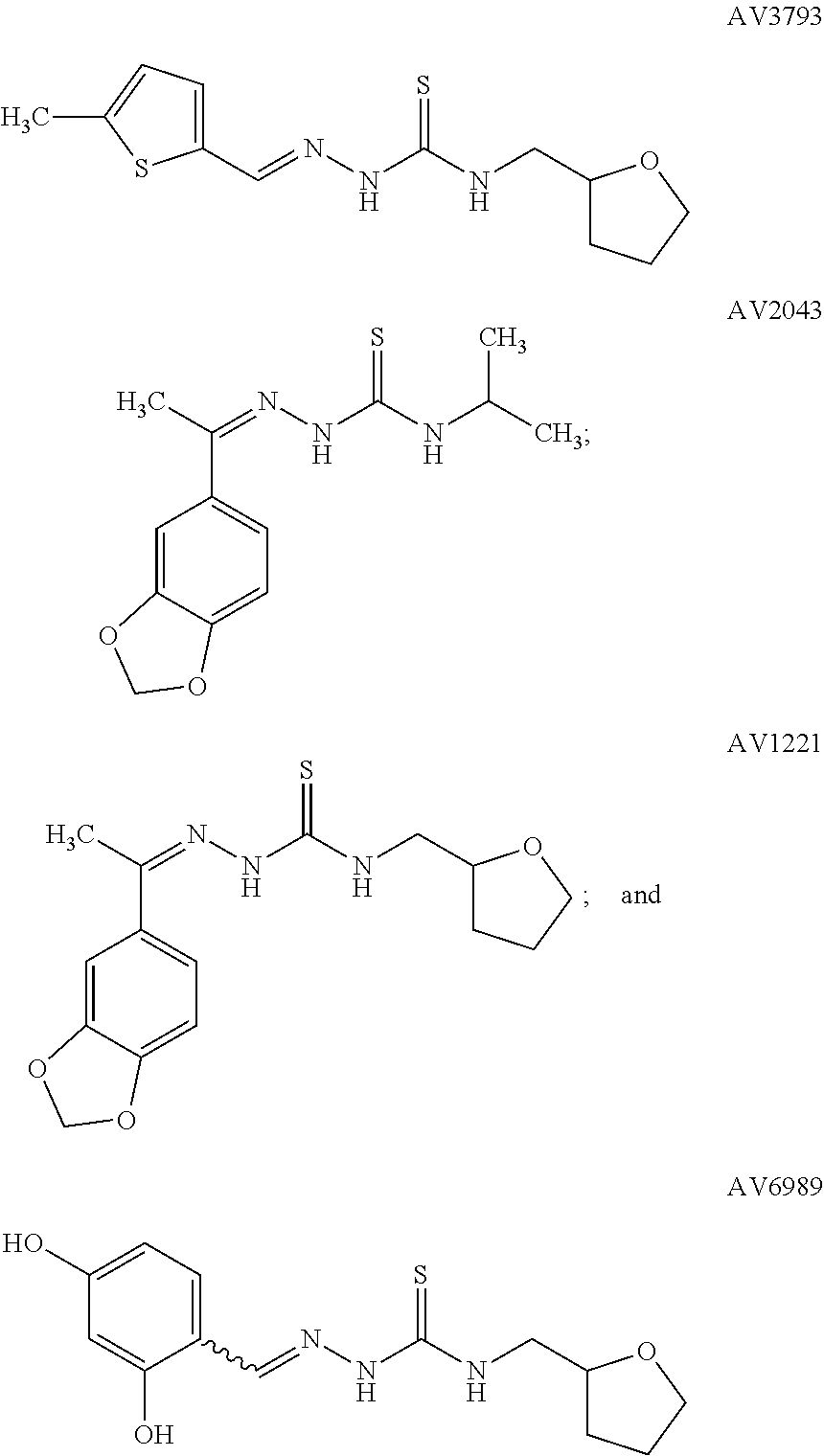
Tyrosinase inhibitors
a technology of tyrosinase inhibitors and inhibitors, which is applied in the field of new compounds and cosmetic formulations, can solve the problems of certain side effects and individuals with sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0107]Mushroom tyrosinase and L-Tyrosine were obtained from Sigma-Aldrich, Inc. (St. Louis, Mo.). The enzyme activity was measured in buffer containing 100 mM phosphate buffer pH 6.8, 5% absolute ethanol, 2 micrograms / milliliter mushroom tyrosinase, and 0.2 mg / ml L-Tyrosine. The reaction (conversion of L-Tyrosine to DOPAchrome) was conducted in triplicates at 25° C. for 30 min, and absorbance was then measured at 492 nm. Kojic Acid was used as a positive control inhibitor in these assays. Percent change in tyrosinase activity relative to vehicle control was calculated. Table 1 shows the effect of treatment with each substance on tyrosinase synthesis in the mushroom tyrosinase assay.
TABLE 1PERCENT CHANGE IN TYROSINASE ACTIVITY COMPARED TO DMSO VEHICLE CONTROLMUSHROOM TYROSINASE ASSAYTyrosinaseTest CompoundConcentrationInhibitionAV69890.001%−36%AV6989 0.01%−75%
example 2
Inhibition of Melanin Production
[0108]The effects of various test compounds on melanin levels were determined by performing assays using B16 melanoma cells. These cells are known to constitutively produce melanin and are a commonly utilized and accepted model system for monitoring the inhibition of melanin synthesis. The B16 mouse melanoma cells were seeded (ATCC, cat. #: CRL-6475) into 96-well tissue culture-treated plates (BD Falcon) and treated with test actives or controls in phenol red free DMEM (Mediatech; cat. #: 17-205-CV) with 2 nanogram / ml of alpha MSH (Fluka; cat #:63605). The cells were examined for their ability to modulate pigment formation. Cells were exposed to diluted test actives or control, where test active had a final concentration of 0.001% or 0.0001%. Tests were performed in 6 replicates each. Following the treatment period (4 days), the level of pigment produced or melanin synthesized was quantified by reading the absorbance at 540 nm using a standard micropl...
example 3
Inhibition of Melanin Production in Human Primary Melanocyte
[0111]The effects of various test compounds on melanin productions were determined by performing assays using Human Epidermal Melanocytes, neonatal, darkly pigmented donor (HEMn-DP). The HEMn-DP cells were seeded (Invitrogen; cat. #: C-202-5C) into 6-well tissue culture-treated plates (BD Falcon) and treated with test actives or controls in Medium 254 (Invitrogen; cat. #: M-254-500) supplemented with 1% HMSG (Invitrogen; cat. #: S-002-5) and 1% P / S. The cells were examined for their ability to modulate pigment formation. Cells were exposed to diluted test actives or control, where test active had a final concentration of 0.001% or 0.0001%. Tests were performed in triplicates each. Following the treatment period (5 days), supernatants were aspirated. The melanin was extracted with 0.5 ml of 2N NaOH for 30 minutes at 80° C. The level of melanin synthesized within the cells was quantified by reading the absorbance at 405 nm us...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| aliphatic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com