Production method of recombinant adenovirus gene vaccine for preventing novel coronavirus
A technology of recombinant adenovirus and gene vaccine, which is applied in the field of biopharmaceutical technology, can solve the problems that cannot meet the requirements of large-scale industrialization of gene therapy products, and achieve the effect of large-scale industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The technical solutions of the present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments.
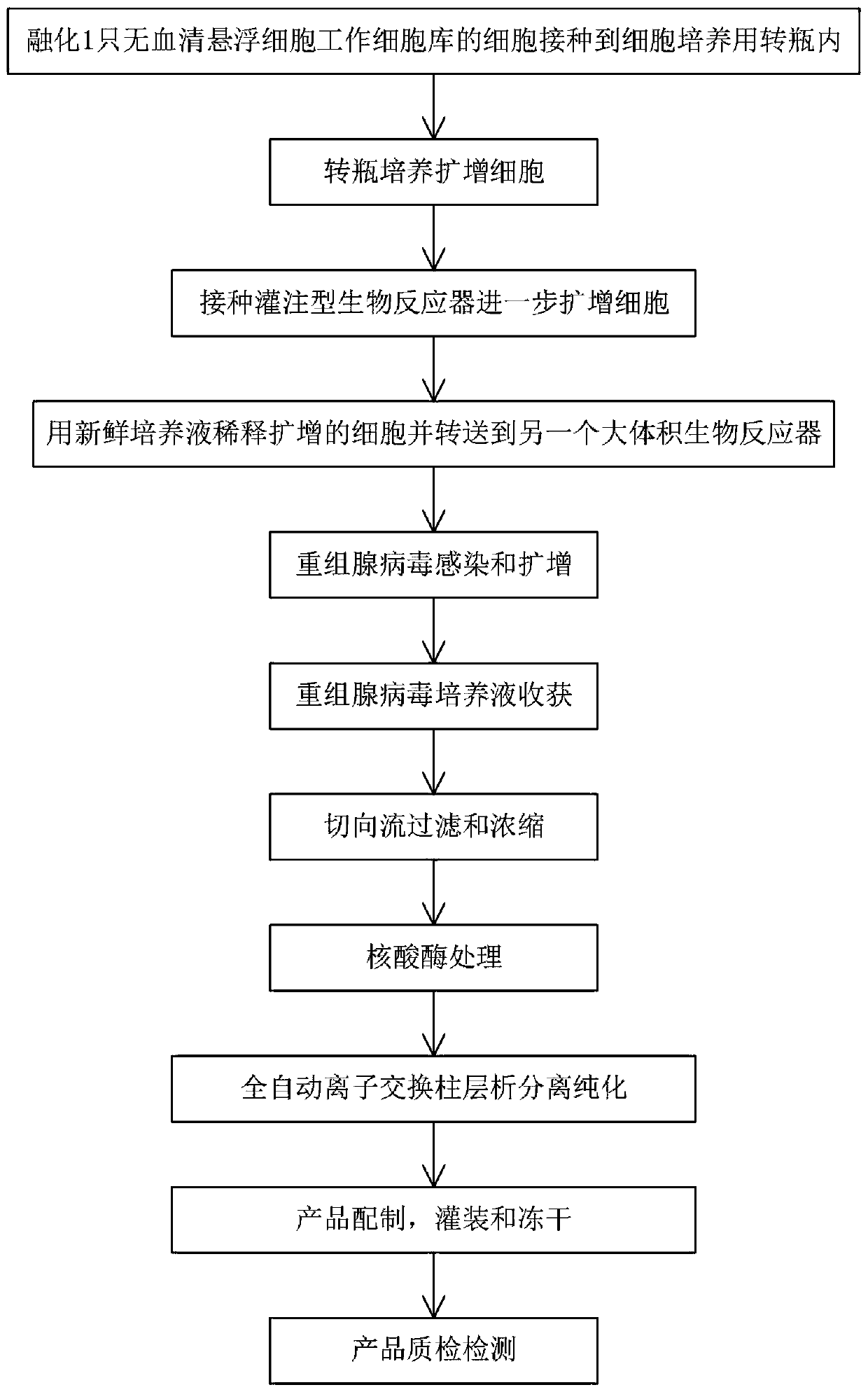
[0028] refer to figure 1 As shown, this embodiment is a production method of a recombinant adenovirus gene vaccine used to prevent new coronaviruses, using serum-free suspension cells to produce recombinant adenovirus, including the following steps:
[0029] 1. Cell expansion
[0030] Take 1 cell tube from the working cell bank of serum-free suspension cells, the cell volume is about 1x10 7 to 1x10 8 . Cells were thawed and inoculated into spinner bottles for cell culture at a seeding density of about 3x10 4 to 1x10 4 / ml. Cultivate in a 5% carbon dioxide incubator at 37°C. Spinner bottles were shaken at 50–150 rpm. Culture cells to a density of approximately 2x10 6 / ml, dilute the cell concentration to 2x10 with fresh medium 5 / ml, and expanded into larger spinner bottles, and continued to culture. Repeat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com