A kind of preparation method of silicon dioxide
A technology of silicon dioxide and carbon dioxide, applied in the directions of silicon dioxide, silicon oxide, chemical instruments and methods, etc., can solve the problems of low reaction efficiency, and achieve the effect of simple operation and large-scale industrialization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] First add 0.07mol diisobutylamine and 0.25mol water into the glass flask, and then pass carbon dioxide into the glass flask to form a homogeneous aqueous solution. A sodium silicate solution with a concentration of 0.75 mol / L was prepared, 20 mL of the sodium silicate solution was mixed with the above aqueous solution, and the reaction was stirred at 70° C. for 20 minutes. The reacted solution is subjected to solid-liquid separation, and the solid silica obtained after separation is properly washed and dried to obtain a white silica powder. The solution obtained by solid-liquid separation was heated to 40°C, allowed to stand still for phase separation, the upper layer of diisobutylamine was recovered, and the lower layer of aqueous solution was evaporated to obtain sodium carbonate.
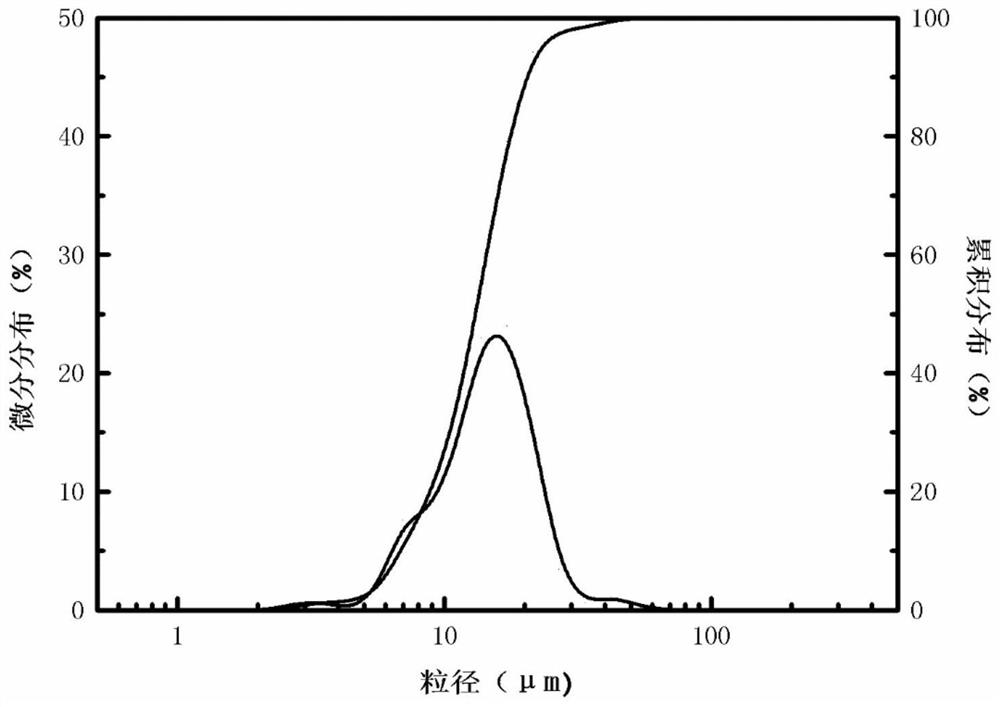
[0030] The yield of the silicon dioxide product obtained in Example 1 is about 95%. Laser particle size analyzer (LS908) measures the average particle size of the product silica to be 13....
Embodiment 2
[0032] First add 0.04mol tripropylamine, 0.03mol dimethylcyclohexylamine and 0.25mol water into the glass flask, and then pass carbon dioxide into the glass flask to form a homogeneous aqueous solution. A sodium silicate solution with a concentration of 0.75 mol / L was prepared, 20 mL of the sodium silicate solution was mixed with the above aqueous solution, and stirred and reacted at a temperature of 70° C. for 15 minutes. The reacted solution is subjected to solid-liquid separation, and the solid silica obtained after separation is properly washed and dried to obtain a white silica powder. Add 0.35 mol of sodium hydroxide to the solution obtained by solid-liquid separation, then let it stand still to separate the phases, recover the upper layer of tripropylamine and dimethylcyclohexylamine, and evaporate the lower layer of aqueous solution to obtain sodium carbonate.
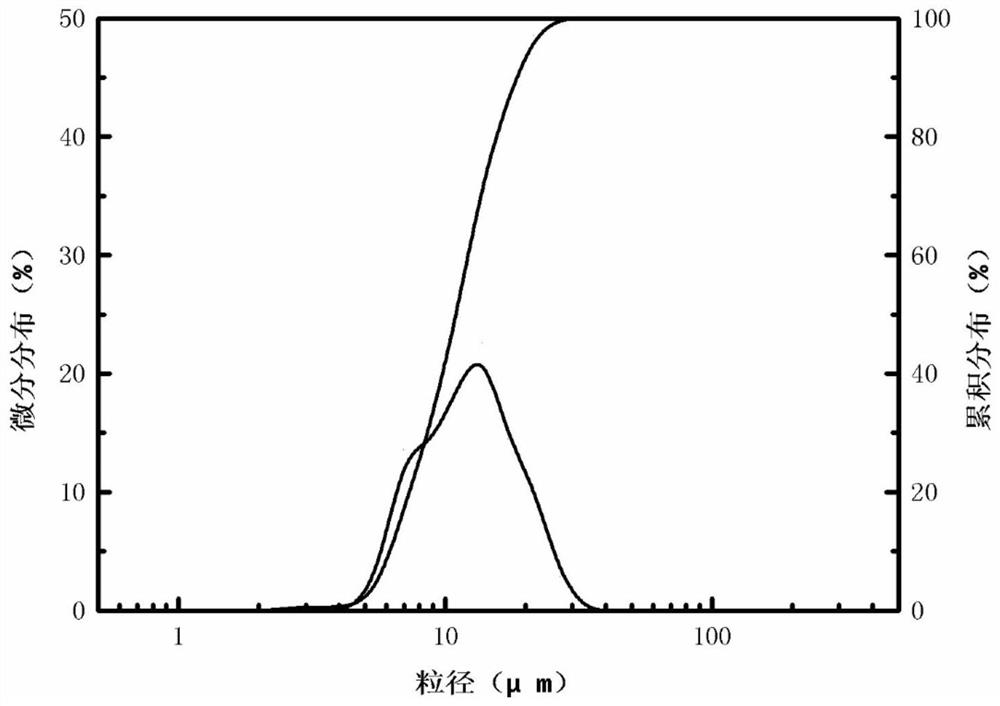
[0033] The yield of the silicon dioxide product obtained in Example 2 is about 95%. Laser particle size ana...
Embodiment 3
[0035] First add 0.07mol 2,6-dimethylpiperidine and 0.25mol water into the glass flask, and then pass carbon dioxide into the glass flask to form a homogeneous aqueous solution. A sodium silicate solution with a concentration of 0.5 mol / L was prepared, 30 mL of the sodium silicate solution was mixed with the above aqueous solution, and stirred and reacted at a temperature of 70° C. for 15 minutes. The reacted solution is subjected to solid-liquid separation, and the solid silica obtained after separation is properly washed and dried to obtain a white silica powder. The solution obtained by solid-liquid separation was heated to 110°C, allowed to stand still for phase separation, the upper layer 2,6-dimethylpiperidine was recovered, and the lower layer aqueous solution was evaporated to obtain sodium carbonate.
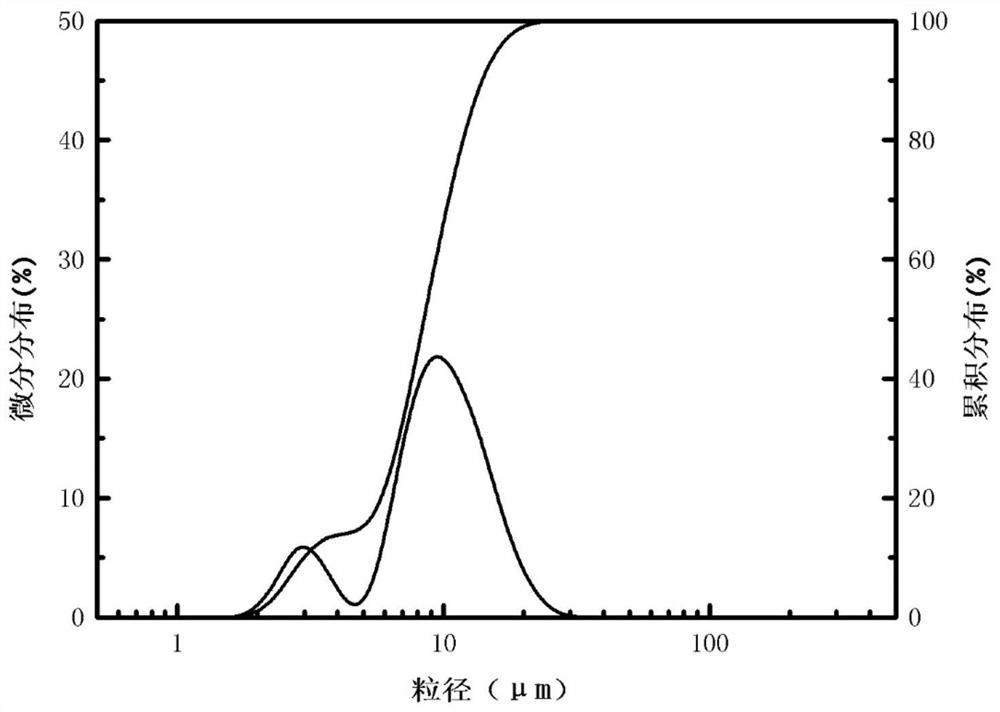
[0036] The yield of the silicon dioxide product obtained in Example 3 is about 92%. Laser particle size analyzer (LS908) measures the average particle size of product ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com