CAR-T cell for treating AIDS-associated lymphoma, and preparation method and application thereof
A tumor cell and cell technology, applied in the direction of genetically modified cells, botany equipment and methods, biochemical equipment and methods, etc., can solve the problems of missed treatment timing, long process, low tolerance, etc., to reduce the risk of GVHD , strong amplification and differentiation capabilities, simple collection process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
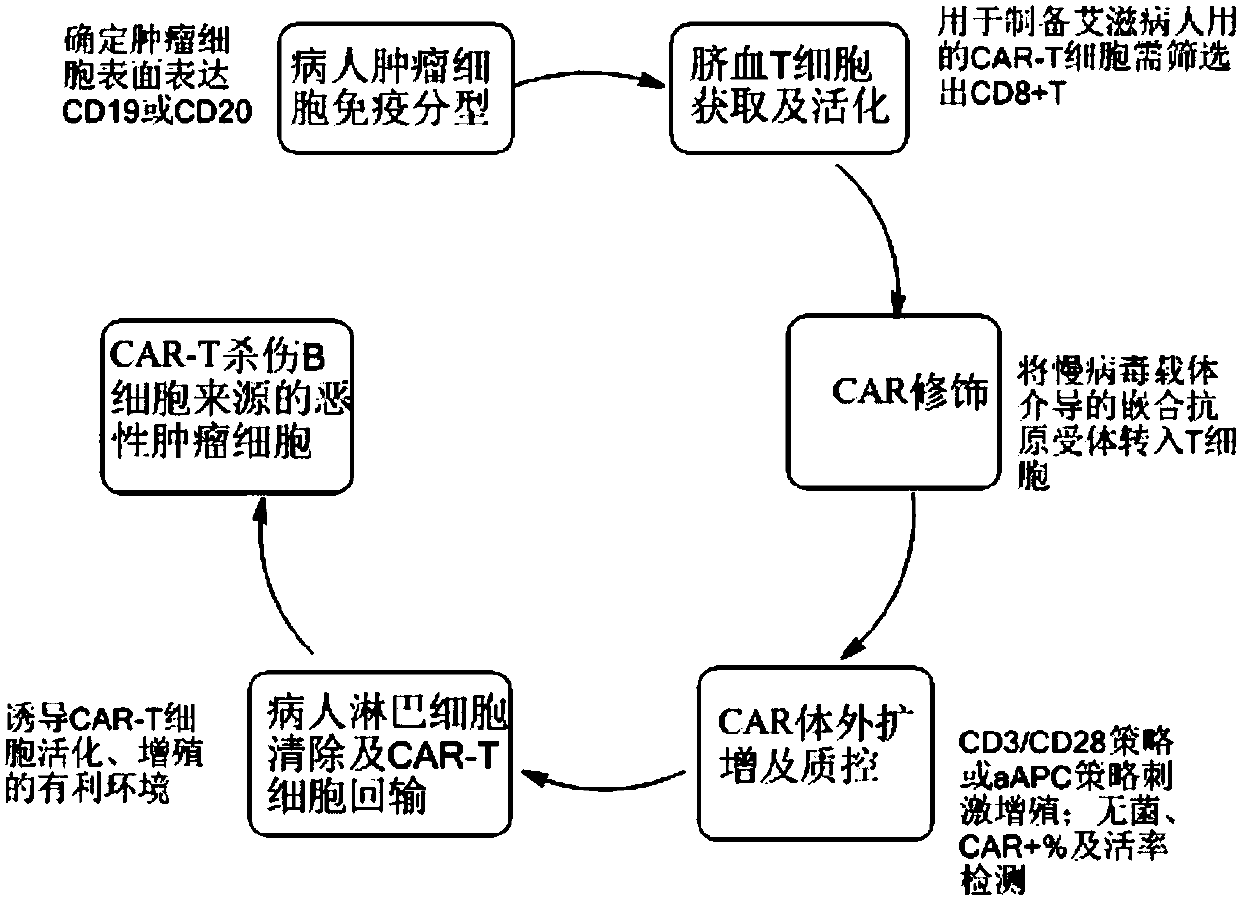
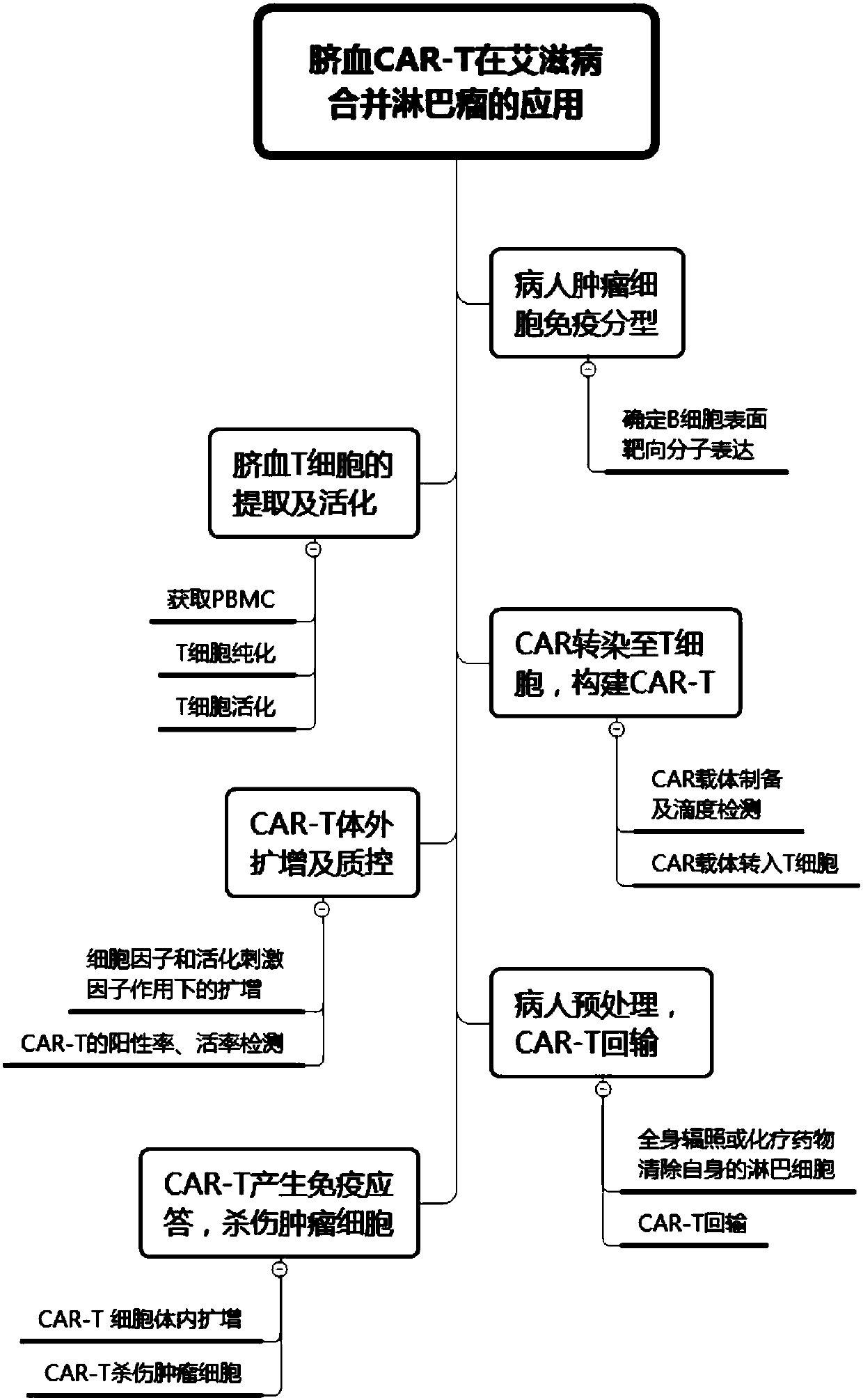
[0046] This embodiment is a preparation step of CAR-T cells targeting CD19, such as figure 1 and figure 2 As shown, the specific steps are as follows:
[0047] 1) Immunotype the patient's tumor cells to confirm the expression of the surface molecule CD19, which can be treated by CD19-CAR-T;
[0048] 2) Obtain umbilical cord blood mononuclear cells by leukapheresis, the specific steps are as follows:
[0049] a) Collecting and screening cord blood from volunteers, wherein the inner wall of the test tube for collecting cord blood is provided with an anticoagulant to prevent cord blood from coagulating, and the screening and testing items include carrying virus or genetic family history;
[0050] b) Centrifuge the collected umbilical cord blood at 1200rmp for 25min to obtain plasma, and add DPBS or normal saline at a volume ratio of 1:1 for dilution;
[0051] c) Add a commercially available lymphocyte separation solution (such as Ficoll separation solution) to the diluted pla...
Embodiment 2
[0061] The CAR-T cells made from umbilical cord blood in Example 1 were compared with the CAR-T cells made from peripheral blood in vitro. The specific experimental steps are as follows:
[0062] The first step: Calcein-AM labeling target cells;
[0063] Dilute Calcein-AM with DMSO to 1 mg / mL; resuspend the target cells with full medium to 1×10 6 Density / mL; add 15 μM Calcein-AM, 37°C, 5% CO 2 Incubate for 30min, mix gently every 10min; centrifuge at 1500rpm, remove supernatant, resuspend with full medium, repeat twice;
[0064] The second step: killing;
[0065] Resuspend the labeled target cells at a density of 5,000-50,000 / mL, take 100 μL and add them to a 96-well plate, add 100 μL of effector cells according to the appropriate ET ratio, and each group has 3 parallels; at the same time, there is a separate group A 6 parallels, only target cells (spontaneous release); 6 parallels in separate group B, only target cells + 2% Triton X-100 (maximum release);
[0066] third s...
experiment example 3
[0070] This example is to verify the safety and effectiveness of the prepared CAR-T cells in vivo.
[0071] First, use AIDS model mice to verify the effectiveness of CAR-T cells. The specific steps are as follows:
[0072] 1) Construction of a humanized mouse AIDS model: After the humanized mouse Scid-hu-Thy / Liv mice were anesthetized, the renal capsule graft was exposed, and HIV virus was directly inoculated into the graft to replicate HIV-1 infection model, and then screen for concurrent lymphoma (volume 100mm 3 Left and right) mice were used as test subjects.
[0073] 2) cell culture;
[0074] T cell culture:
[0075] Count and determine the viability of T cells, resuspend with normal saline after centrifugation, and adjust its concentration to 5×10 5 pc / mL, total up to 6×10 5 indivual.
[0076] CAR-T cell culture:
[0077] Determine the CAR content percentage of CAR-T cells, count and measure the activity rate, resuspend with normal saline after centrifugation, adju...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com