Method for preparing human dendritic cell vaccine
A technology of dendritic cells and vaccines, applied in the field of cellular immunology, to achieve the effect of simple process, low cost, and easy large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
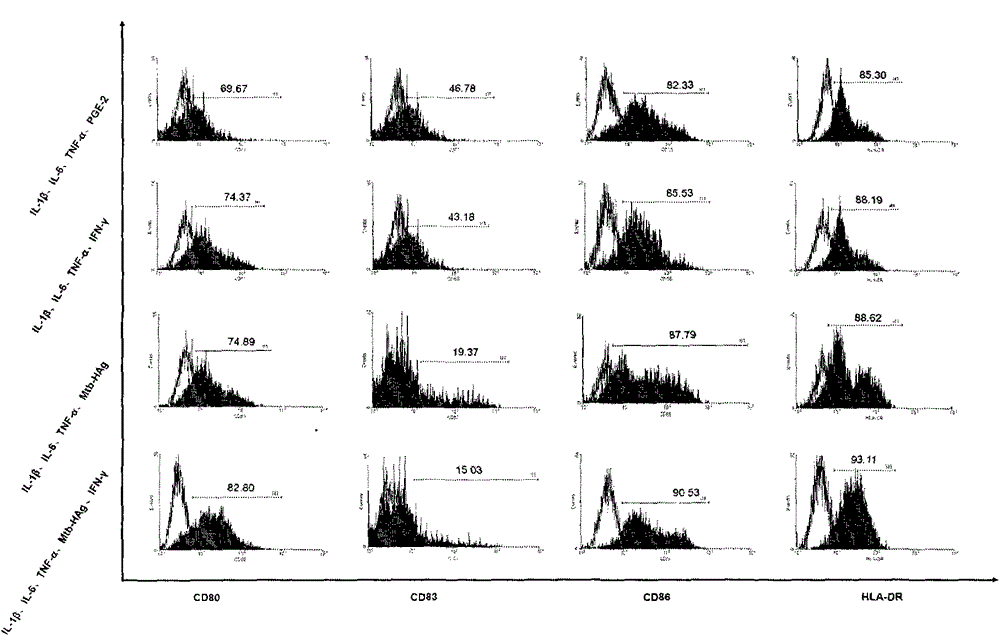
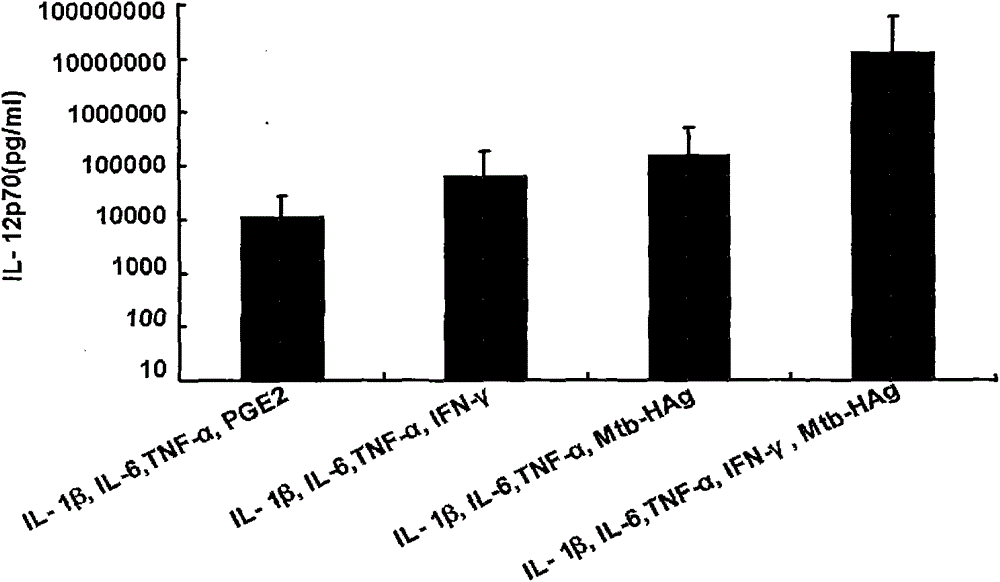
[0024] Example 1 Preparation of human dendritic cell vaccine and detection of maturity and IL-12 secretion
[0025] The first step: further separate and obtain mononuclear cells from the isolated PBMCs, including the following steps:
[0026] (1) 50 ml of peripheral venous blood was collected from the patient, and mononuclear cells were obtained by density gradient centrifugation of Ficoll-diatrizoate glucosamine. The specific steps are: 1500 rpm, centrifuge for 10 minutes, absorb the upper plasma layer, inactivate at 56°C for 30 minutes and centrifuge for later use, dilute the precipitated blood cells with normal saline, and divide human lymphocyte separation medium and diluted blood at a ratio of 1:2 The proportion of the mixture was added to the centrifuge tube, 2000 rpm, centrifuged for 20 minutes, carefully sucked the buffy coat layer, washed twice with normal saline, the speeds were 1600 rpm, 1300 rpm, and centrifuged for 7 minutes to obtain the peripheral Blood mononuc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com