Dendritic cell vaccine carrying recombinant human HSP70 polypeptide complexes, preparation method and application
A technology of dendritic cells and polypeptide complexes, applied in the direction of drug combinations, medical preparations containing active ingredients, carrier-bound antigen/hapten components, etc., to achieve the effects of clear amino acid sequence, flexible design, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
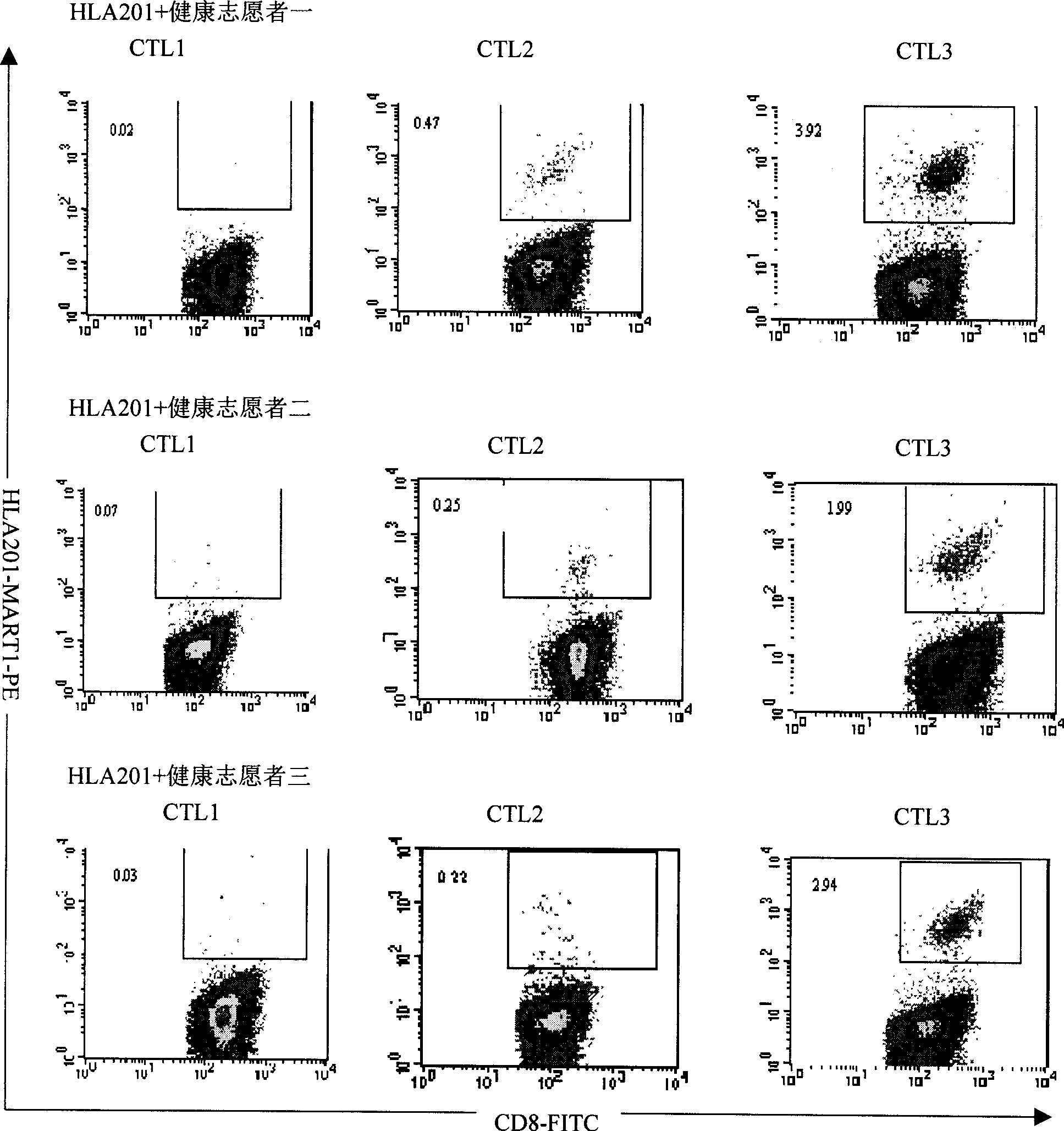
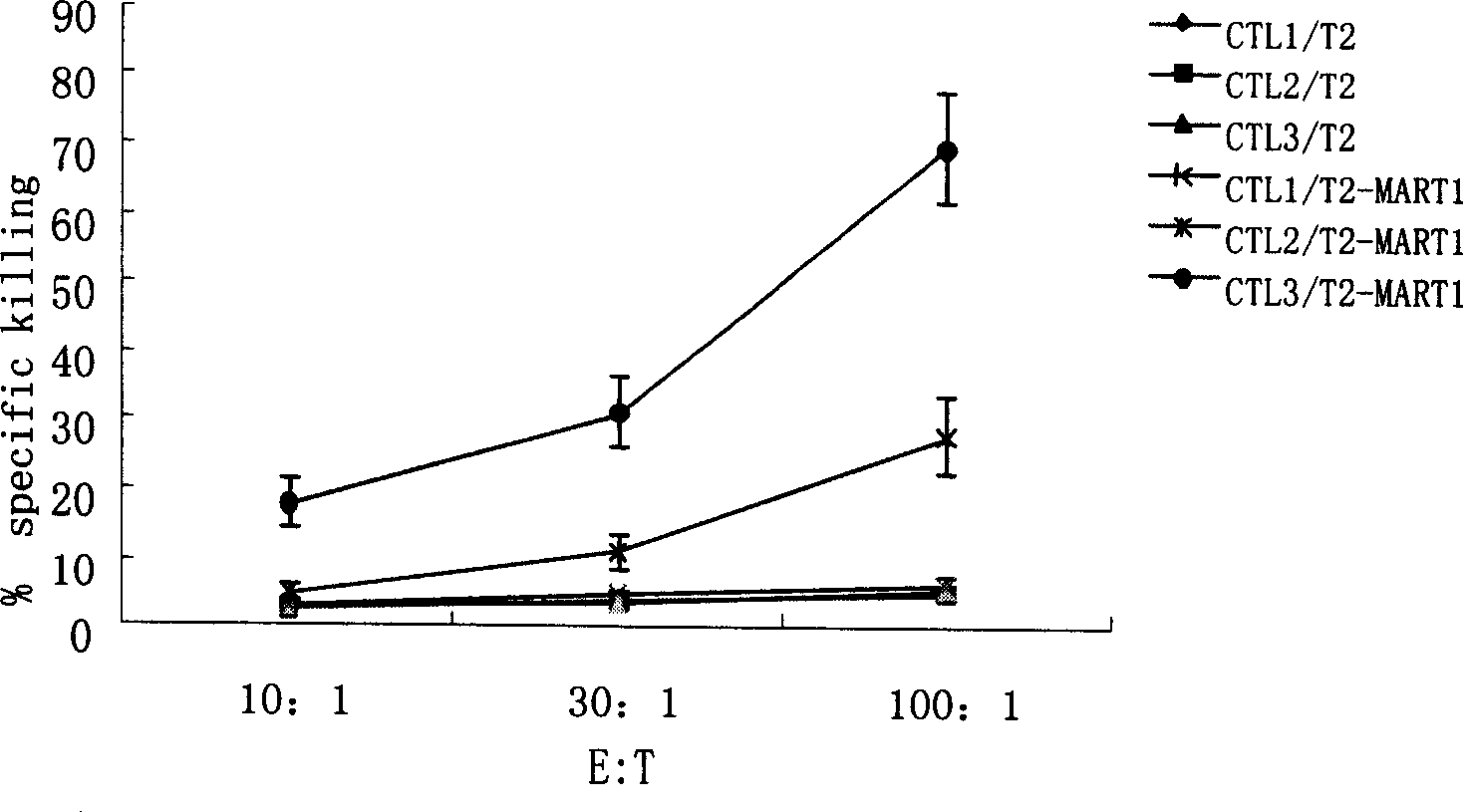
[0085]Example 1: Dendritic cell vaccine loaded with four HSP70-melanoma antigen polypeptide complexes
[0086] 1. Preparation of melanoma antigen polypeptide and rhHSP70 protein
[0087] The known HLA-A201-restricted melanoma antigen polypeptide fragments were artificially synthesized, and the names and sequences were GP100: IMDQVPFSV; MART1: AAGIGILTV; MAGE3: FLWGPRALV and Try: YMDGTMSQV. Fully dissolved in DMSO, the peptide concentration was 5 mg / mL; rhHSP70 was dissolved in PBS, the concentration was 0.1 μg / mL.
[0088] 2. Preparation of rhHSP70-polypeptide complex
[0089] Take 5μg (5μl) polypeptide and 10μg (100μl) rhHSP70 polypeptide respectively in 1.5mL EP tube, add 400μl containing 10nM ATP, 1mM KCl, 2mM MgCl 2 Mix well and incubate at 20°C for 40 minutes, add 100nM ADP and continue to incubate at 20°C for 40 minutes. Transfer the solution to a 0.5mL microdialysis tube (containing a 14kD molecular weight ultrafiltration membrane) overnight, remove unbound polypepti...
Embodiment 2
[0103] Example 2: Liver cancer dendritic cell vaccine loaded with two kinds of HSP70-liver cancer antigen polypeptide complexes
[0104] 1. Antigen polypeptide synthesis: Most patients with primary liver cancer highly express alpha-fetoprotein (AFP) and are combined with chronic persistent HBV infection. For this type of liver cancer patients, AFP and HBV can be used as target antigens, such as AFP: GVALQTMKQ; HBsAg: FLLTRILTI; Polypeptide dissolution and concentration are the same as in Example 1;
[0105] 2. Preparation of rhHSP70-polypeptide complex: the operation steps are the same as in Implementation 1, and two complexes of HSP70-AFP and HSP70-HBsAg are prepared;
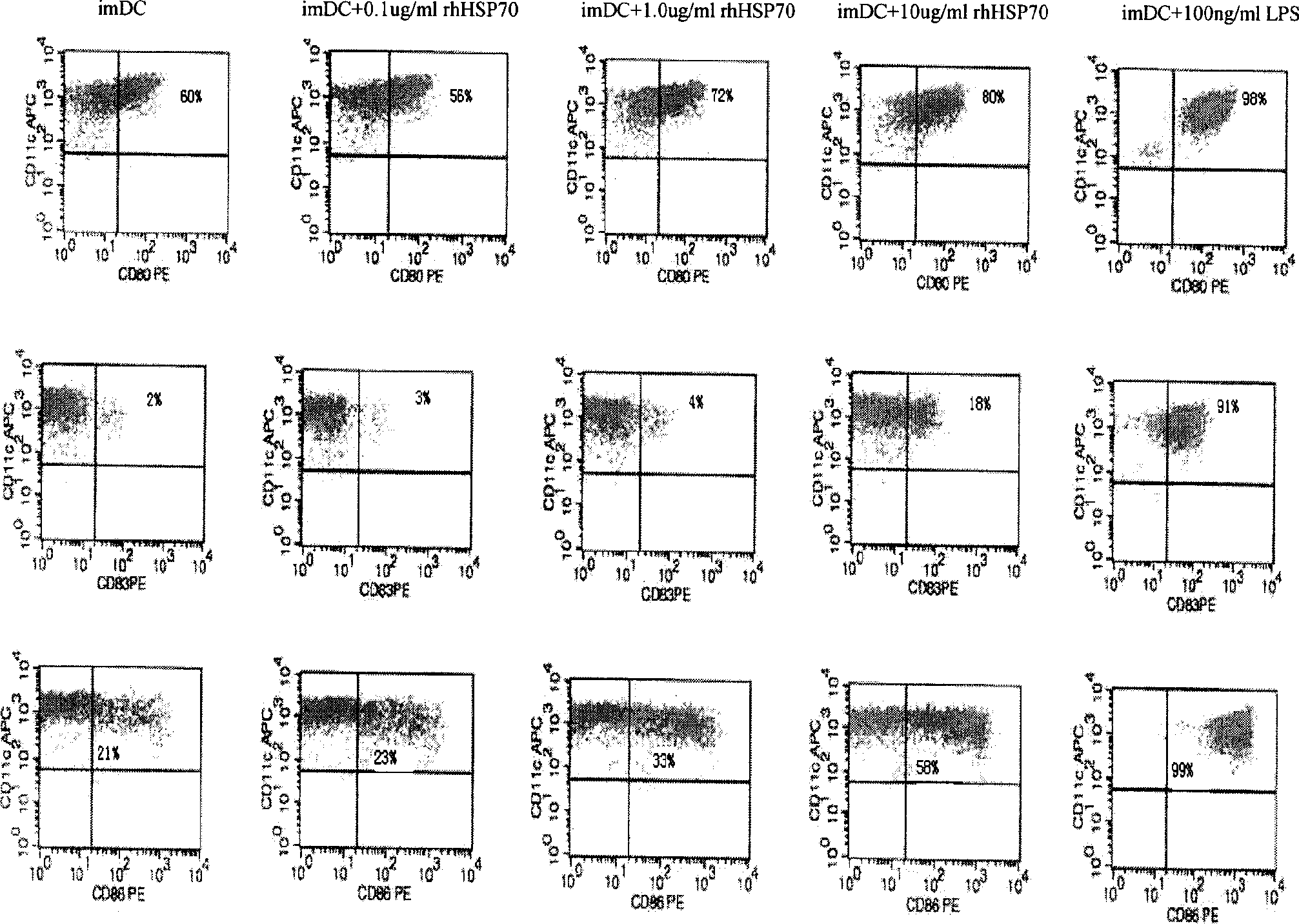
[0106] 3. Induction of dendritic cells in vitro
[0107] Collect fresh heparin anticoagulated primary liver cancer patients (HLA-A201 + ) peripheral blood or HLA-A201 + Peripheral mononuclear cells (PBMCs) were obtained from the peripheral blood of normal healthy persons by Ficoll density gradient centrifug...
Embodiment 3
[0112] Example 3: Dendritic cell vaccine loaded with two kinds of HSP70-breast cancer antigen polypeptide complex
[0113] 1. Antigen peptide synthesis: Most breast cancer patients highly express MUC1 and Her2-neu related antigens; for this type of breast cancer patients, this can be used as a target antigen to synthesize peptides, such as MUC1: SLADPAHGV; Her2-neu: KIFGSLAFL; peptide dissolution and Concentration is with embodiment 1;
[0114] 2. Preparation of rhHSP70-polypeptide complex: the operation steps are the same as in Implementation 1, and two complexes of HSP70-MUC1 and HSP70-Her2-neu are prepared;
[0115] 3. Induction of CD34-DCs in vitro
[0116] HLA-201+ breast cancer patients undergo hematopoietic mobilization with GM-CSF, collect mononuclear cells (PBMCs) with a cell harvester, add CD34 monoclonal antibody magnetic beads to separate and purify CD34+ hematopoietic precursor cells (purity>90%), and suspend them In X-VIVO medium containing 5% (v / v) mixed human...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com