Preparation method of breast cancer-specific epitope polypeptide-loaded dendritic cell vaccine and kit thereof
A technology of dendritic cells and antigenic epitopes, applied in animal cells, vertebrate cells, blood/immune system cells, etc., can solve the problems of cancer recurrence, poor prognosis, and inability to obtain patients, and achieve a radical cure for recurrence and metastasis , the effect of overcoming tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
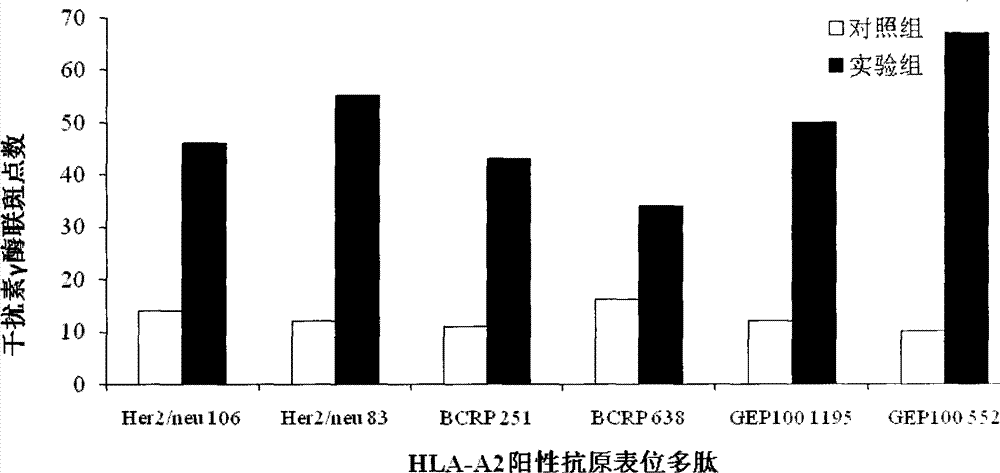
[0048] Preparation example 1 Human Her2 / neu, BCRP and GEP100 protein HLA-A2010 positive epitope peptide prediction
[0049] The bioinformatics and molecular analysis system (BIMAS) was used to predict the binding of HLA polypeptides, and the HLA epitope polypeptides that specifically bind to human Her2 / neu, BCRP and GEP100 proteins were screened, and 6 kinds of HLA-A0201 binding affinity over 100 were selected. The amino acid sequence of is shown in Table 1:
[0050]
[0051]
[0052] Preparation Example 2 Dendritic cell acquisition
[0053] Take 100 mL of peripheral blood by Ficoll-Hypaque density gradient centrifugation to obtain mononuclear cells. Peripheral blood mononuclear cells were resuspended in RPMI1640 medium and added to a 6-well plate to adhere. At 37℃, 5% CO 2 After 90 minutes of incubation in the incubator, the non-adherent cells were washed and collected for induction of CIK cells. Adherent cells were induced by adding complete medium RPMI1640, containing 5% autolo...
Embodiment 3
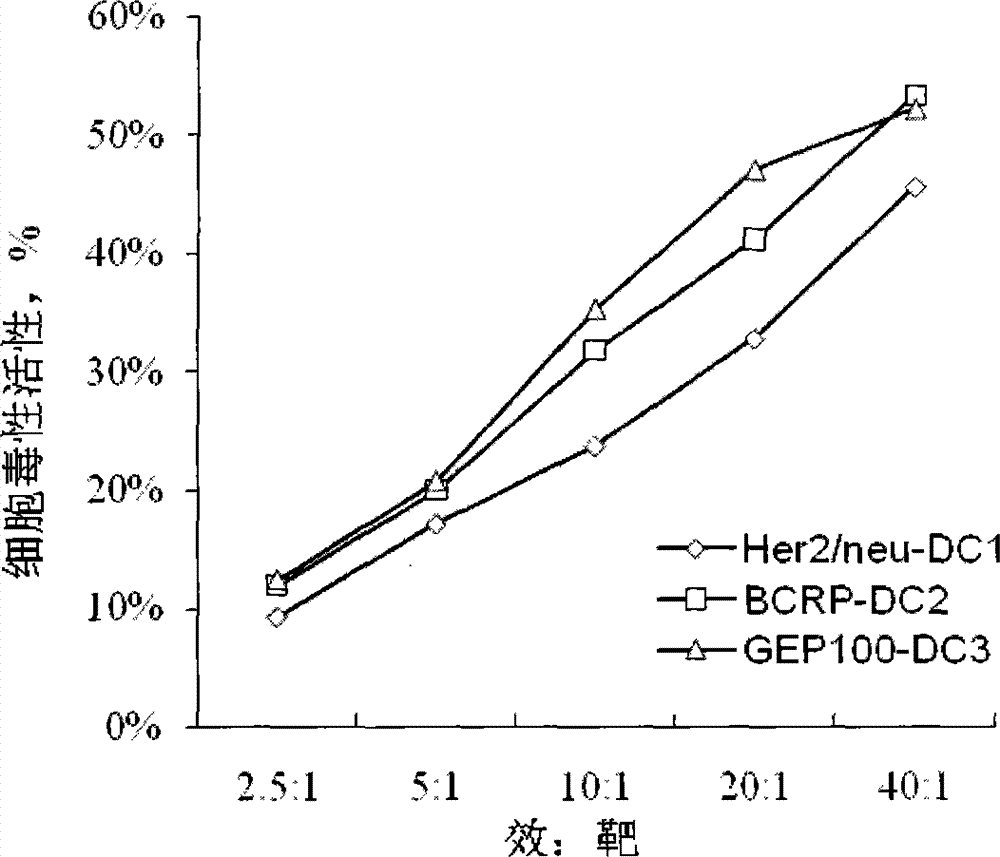
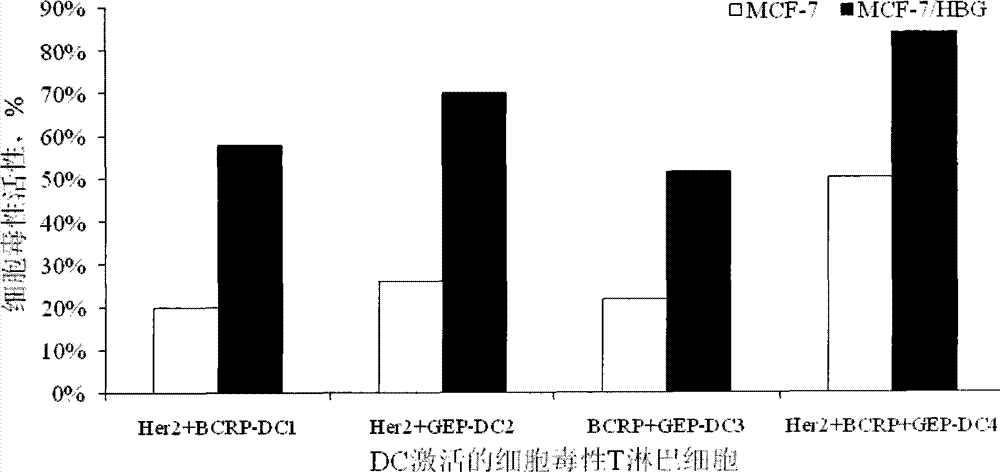
[0054] Example 3 DCs loaded with Her2 / neu, BCRP and GEP100 protein epitope polypeptide
[0055] After 5 days of cultivation, 3×10 6 Immature DCs use the HER2 / NEU, BCRP and GEP100 protein SEQ ID NO. 1-6 of the HER2 / NEU, BCRP and GEP100 protein SEQ ID NO. 1-6 polypeptide composition with more than one epitope or three epitope polypeptide compositions of the three antigens At 37℃, 5% CO 2 Load in the incubator for 2 hours. The antigen-loaded DCs were harvested by centrifugation (10min, 1000rpm). The DCs obtained were washed 3 times with normal saline, and then resuspended in normal saline to a concentration of 3×10 6 Mature DC / mL, and adding human albumin with a final concentration of 2% by mass to volume, thereby preparing a DC vaccine loaded with a composition of Her2 / neu, BCRP and GEP100 protein epitope polypeptide.
Embodiment 1
[0056] The Her2 / neu, BCRP and GEP100 protein SEQ ID NO. 1-6 of the polypeptide composition of more than one epitope in SEQ ID NO. 1-6 described in Example 1 load dendritic cells through CD86-PE, CD80-PE, CD40-FITC, CD83 -PE, CD11c-FITC and HLA-DR-PerCP (BD company) staining, flow cytometry to detect DC phenotype changes after loading. The test results showed that the phenotype of the prepared DC was CD11c+ / HLA-DR+ 95.0%, CD11c+ / CD83+ 79.8%, CD86+ / HLA-DR+ 89.9%, CD80+ / HLA-DR+ 90.5% and CD40+ / HLA-DR+ 85.8%, in line with the specific phenotype of DC cells, and high expression of costimulatory molecules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com