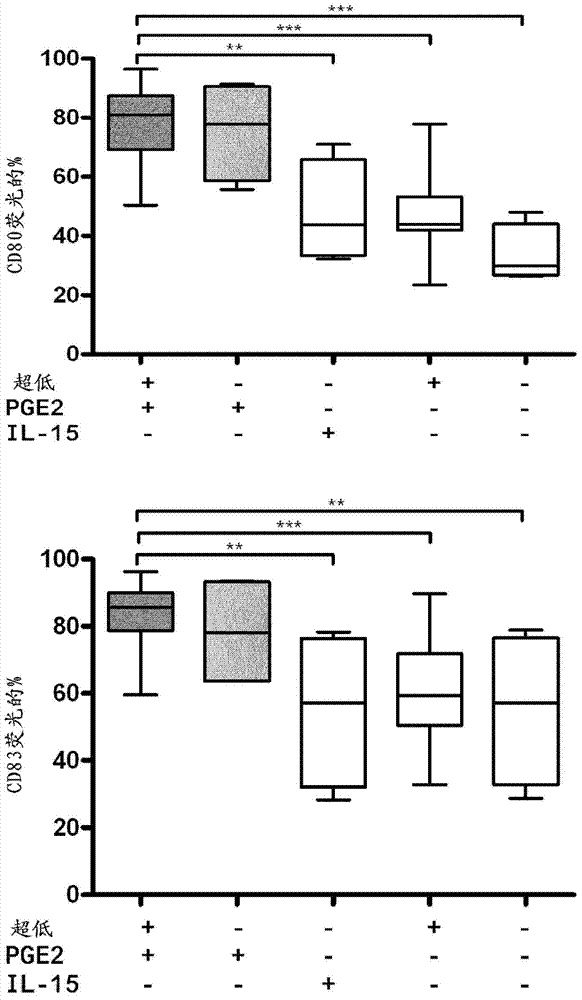
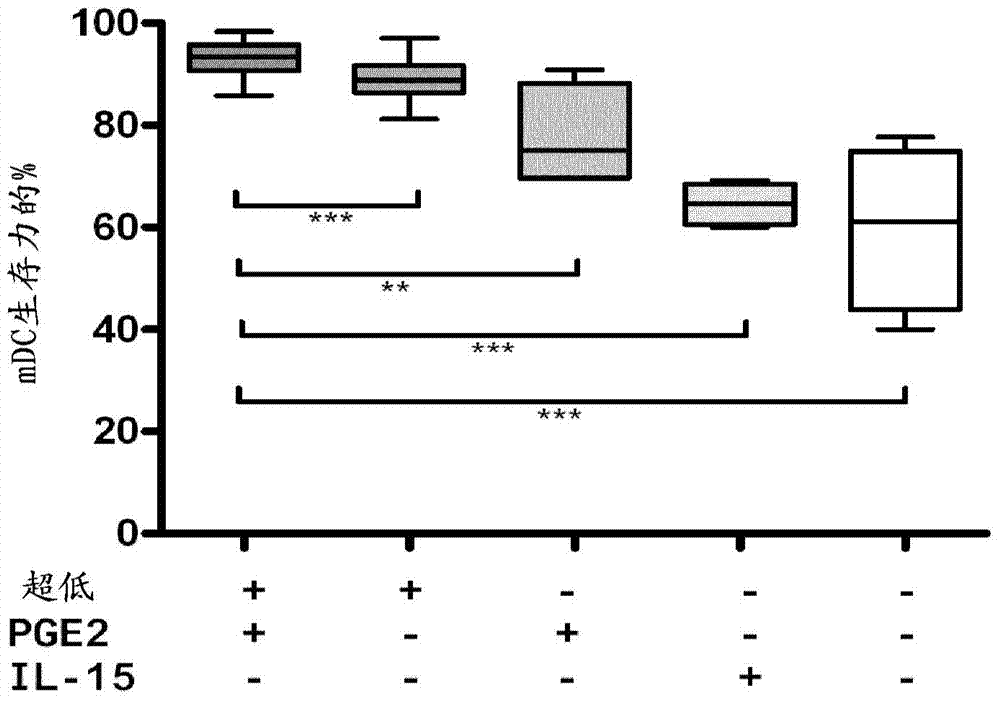
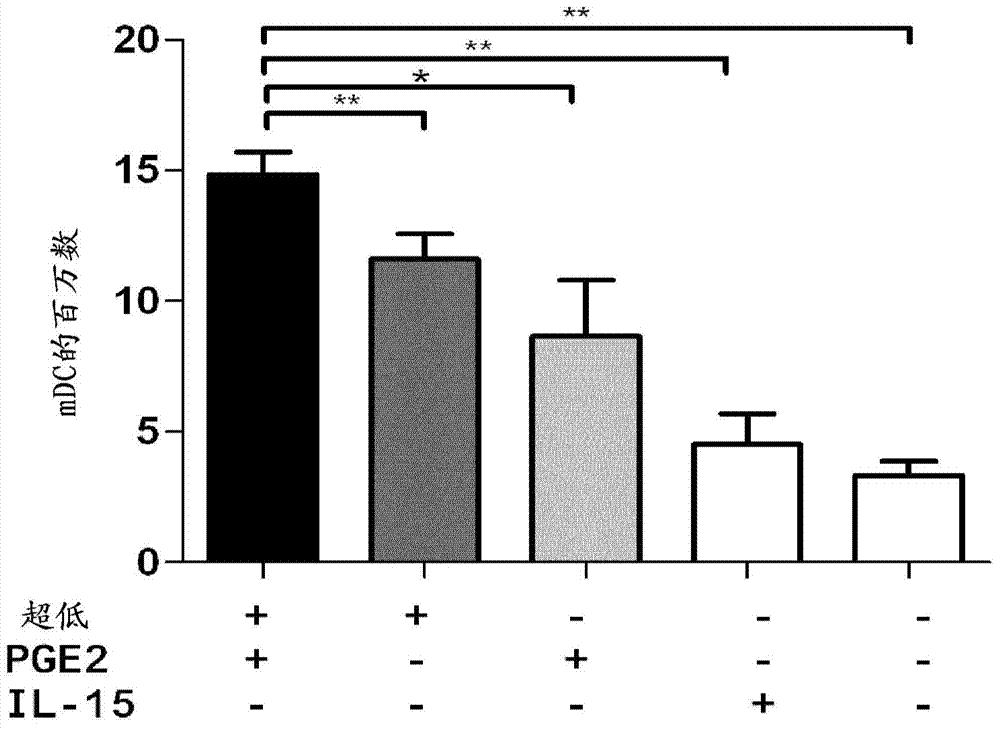
Method for the preparation of dendritic cell vaccines
A technology of dendritic cells and vaccines, applied in the field of treatment of infectious diseases, can solve problems such as inconsistent results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0211] Ex vivo generation of monocyte-derived dendritic cells (MDDCs)
[0212] 150 mL of fresh blood was drawn from a donor subject carrying HIV. Then by Ficoll density gradient (Accuspin , Sigma-Aldrich Corp., Saint Louis, MO, US) isolated peripheral blood mononuclear cells (PBMC) from blood. The resulting solution was centrifuged at 1200 rpm for 5 minutes.
[0213] The suspension was divided into 18 mL aliquots. Pour these aliquots into a 75cm 2 attached culture flask (Corning Inc., Corning, NY, US) and placed in a humidified 5% CO at 37°C 2 Atmosphere incubator for 2 to 3 hours. Unattached cells (lymphocytes) were separated by aspiration. The attached cells were mostly monocytes.
[0214] The attached cells (monocyte layer) were washed 4 times with 15 mL of X-VIVO10 (cGMP, Biowhittaker Inc., Walkersville MD, US) prewarmed at 37°C. Carefully agitate the solution to remove possible lymphocyte contamination due to gravity deposition without dislodging attached monocytes...
Embodiment 2
[0219] Maturation of autologous MDDCs was performed in flasks with attachment surfaces and
[0220] Pulse with inactivated HIV-1
[0221] After culturing for 5 days, 10.5 million MDDCs obtained in Example 1 were centrifuged at 2000 rpm for 5 minutes. Resuspend the pellet in 2.8 mL of basal medium. See Example 1. Add 0.2 mL of an aliquot of inactivated HIV that had been previously resuspended in X-VIVO15 medium containing >10 8 Copies of HIV-1 RNA. Place the cells in a 75 cm vertical and slightly inclined position 2 on a culture flask with an attachment surface. 1000 IU / mL IL-4 and 1000 IU / mL recombinant human (rh)GM-CSF (cGMP quality CellGenix GmbH, Freiburg, DE) were added to each flask and the cells were incubated at 37°C.
[0222] After incubation, add 22 mL of basal medium with GM-CSF and IL-4 at 1000 IU / mL and cytokines IL-6, TNF-α and IL-1-β at 1000 IU, 1000 IU and 300 IU per mL, respectively (cGMP quality, CellGenix GmbH, Freiburg, DE). Cells were incubated in t...
Embodiment 3
[0226] Maturation of autologous MDDCs in ultra-low attachment flasks and
[0227] Pulse with inactivated HIV-1
[0228] After 5 days of culture, 10.5 million MDDCs were centrifuged at 2000 rpm for 5 minutes. Resuspend the pellet in 2.8 mL of basal medium. See Example 1. Add 0.2 mL of an aliquot of inactivated HIV that had been previously resuspended in X-VIVO15 medium containing >10 8 Copies of HIV-1 RNA. Place the cells in a 75 cm vertical and slightly inclined position 2 culture flasks with ultra-low attachment surfaces ( , Cat. No. 153814, Cultek, SLU, Madrid, ES). Add 1000 IU / mL IL-4 and 1000 IU / mL recombinant human (rh) GM-CSF (cGMP quality CellGenix GmbH, Freiburg, DE) to each flask and incubate the cells at 37 °C with the flask in a slightly inclined position for 2 to 4 Hour.
[0229] After incubation, 22 mL of basal medium was added with 1000 IU / mL of GM-CSF and IL-4 and cytokines IL-6, TNF-α and IL-1- Beta maturation mixture. Cells were cultured in the medi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com