Multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses)
A hepatitis C virus and dendritic cell technology, applied in the fields of virology and immunology, can solve the problems of lack of protective antibodies, insufficient understanding of pathogenesis, and inability to prevent patients from reinfection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
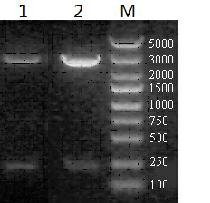
[0027] Example 1. Expression of recombinant adenovirus:
[0028]1. Construction of recombinant adenovirus expression plasmids AD-sequence 1 and AD-sequence 2: Sequence 1 in pGH and Sequence 2 in pGH were synthesized by Generay Biotech (Shanghai). Sequence 1 is HCV CTL epitope NS4B (1793-1801) SMMAFSAAL and P7 (774-782) AAWYIKGRL plus HCV Th epitope NS3 (1248-1261) GYKVLVLNPSVAAT in series, the middle of the epitope is connected by AAY that promotes the presentation of the epitope , using both pichia yeast biased codons and Escherichia coli biased codons to design genes, and artificially synthesize HCV CTL bi-epitope gene tandem. Sequence 2 is a positive control, including HCV CTL epitope Core(35-44)YLLPRRGPRL, Core(132-140)DLMGYIPLV and HCV Th epitope NS3(1248-1261)GYKVLVLNPSVAAT. Sequences 1 and 2 are added with GFP and FLAG tags at the same time, which is beneficial to Western Blot detection of the expression of the target polypeptide. Double digestion with restriction end...
Embodiment 2
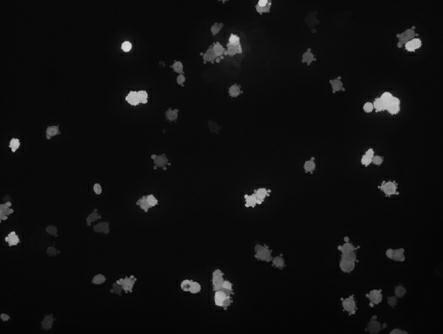
[0030] Example 2. Culture of dendritic cells
[0031] 1. Cultivate dendritic cells: Whole blood comes from healthy donors. Peripheral blood mononuclear cells (PBMC) are separated by Ficoll-Hypaque density gradient centrifugation. CD14+ mononuclear cells are separated and collected according to the CD14 MicroBeads humanmonocyte kit (MACS) operating instructions. cells, the cell purity was 96.32%. Adjust the density of CD14+ monocytes to 1×10 with serum-free medium X-VIVO15 6 Inoculate cells / mL in 24-well plate, incubate at 37°C in 5% CO2 for 5 days, change half of the medium every other day, and add GM-CSF (1000U / ml) and IL-4 (1000U / ml) from the 5th day Mature dendrites can be induced by maturation-inducing factors rTNF-α (10ng / mL), IL-1β (10 ng / mL), IL-6 (10 ng / mL), and PGE2 (1μg / mL) for 2 days shaped cells. Observed under an inverted microscope, after 1 day of culture, most of the cells still adhered to the wall and arranged in clusters, round in shape, with smooth cell me...
Embodiment 3
[0036] Example 3. Detection of Immunogenicity of Dendritic Cell Vaccines
[0037] 1. Mixed lymphocyte culture: DCs infected with recombinant adenovirus and not infected with recombinant adenovirus were collected as stimulator cells. Stimulate cells at 1×10 3 / hole, 5×10 3 / hole, 1×10 4 Each well was inoculated in a 96-well culture plate, and each group had three replicate wells; CD14-PBMC were used as effector cells, and 1×10 5 At the same time, only the effector cells were added as the negative control, and only the culture medium was added as the blank control, and the final volume of each well was 200 μl. The mixed lymphocytes were cultured under the conditions of 5% CO2 and 37°C. After 4 days, 20 μl / well of MTS solution was added, and the incubation was continued for 4 hours. The enzyme-linked immunoassay analyzer detected the A450 value at a wavelength of 450 nm, and the results were expressed as the average value of 3 wells. Stimulation index (SI)=experimental group-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com