Preparation method of dendritic cell (DC) vaccine loaded with autologous tumor associated holoantigen
A technology of autologous tumor antigen and dendritic cells, applied in the field of preparation of dendritic cell vaccines, can solve the problems of weak antigenicity, limited clinical efficacy, and lack of antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0153] The embodiments of the present invention will be further described below in conjunction with the accompanying drawings, but not as a limitation to the present invention. The scope of protection of the present invention is based on the content recorded in the claims. Any equivalent technical means made according to the description of the present invention is replaced. Without departing from the protection scope of the present invention.
[0154] The overall technical concept of this embodiment is:
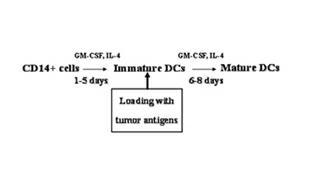
[0155] A preparation method of a dendritic cell vaccine loaded with autologous tumor-associated whole antigens, comprising the following process steps:
[0156] A. Preparation of autologous tumor-associated whole antigen;
[0157] B. Taking and separating and culturing DC cells;
[0158] C. Impulse DC cells with autologous tumor-associated whole antigen, mature DC cells and prepare autologous tumor antigen-specific DC vaccine.
[0159] Concrete technical solution and proce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com