Method for preparing dendritic cell vaccine
A technology of dendritic cells and vaccines, applied in the field of preparation of dendritic cell vaccines, can solve the problems of poor specificity, low loading efficiency, and low targeting, and achieve high loading efficiency, no immune tolerance, Sensitive and specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Preparation of DCs derived from umbilical cord blood
[0019] (1) Separation of umbilical cord blood mononuclear cells:
[0020] Cut the umbilical cord blood bag, measure the umbilical cord blood with a 50ml syringe, dilute the blood with an equal amount of compound electrolyte injection; slowly add the diluted cell suspension to the Ficoll at a ratio of 2:3 between the Ficoll separation medium and the cell suspension The upper layer of the separation solution; centrifuge, 900g, 25min; absorb the cells near the buffy coat at the boundary, and resuspend in the compound electrolyte injection; centrifuge 400g, 8min; absorb the supernatant, and resuspend the cell pellet in the compound electrolyte injection; centrifuge 400g, 8min; Aspirate the supernatant, resuspend the cell pellet and add 10ml of culture medium, and then count; culture DC according to the counting results.
[0021] (2) DC adherent culture
[0022] Use RPMI-1640 medium containing 1% FBS to adjus...
Embodiment 2
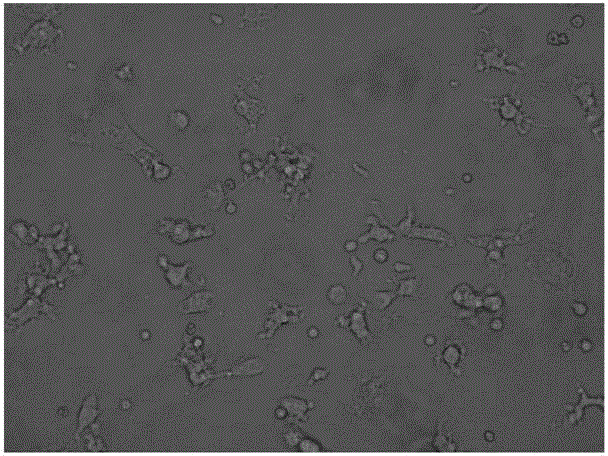
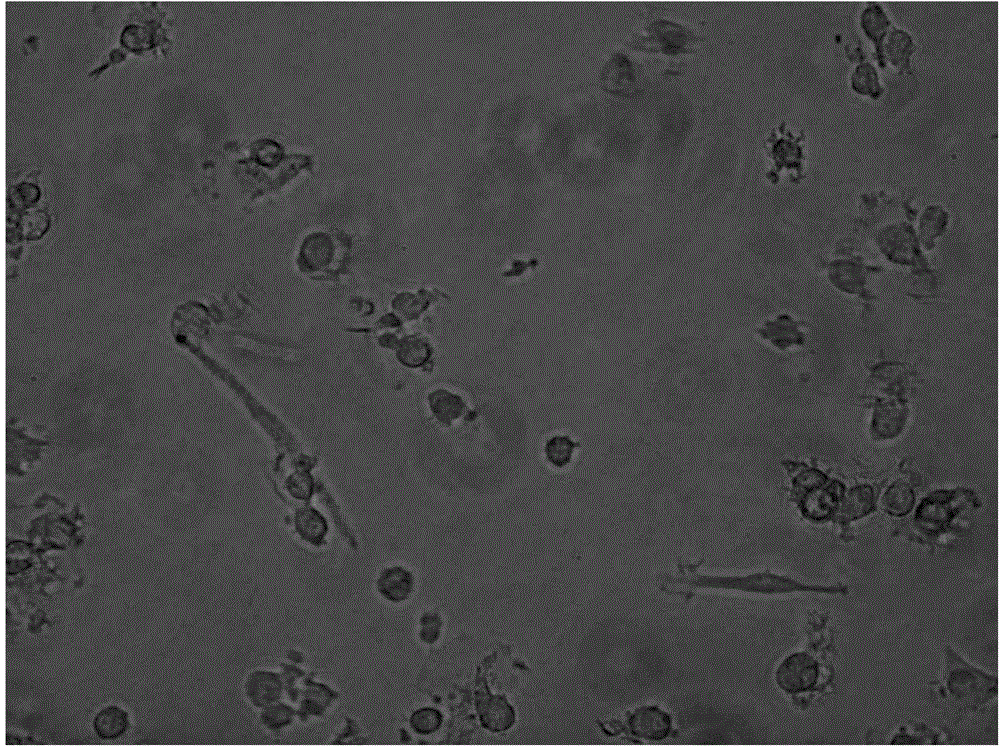
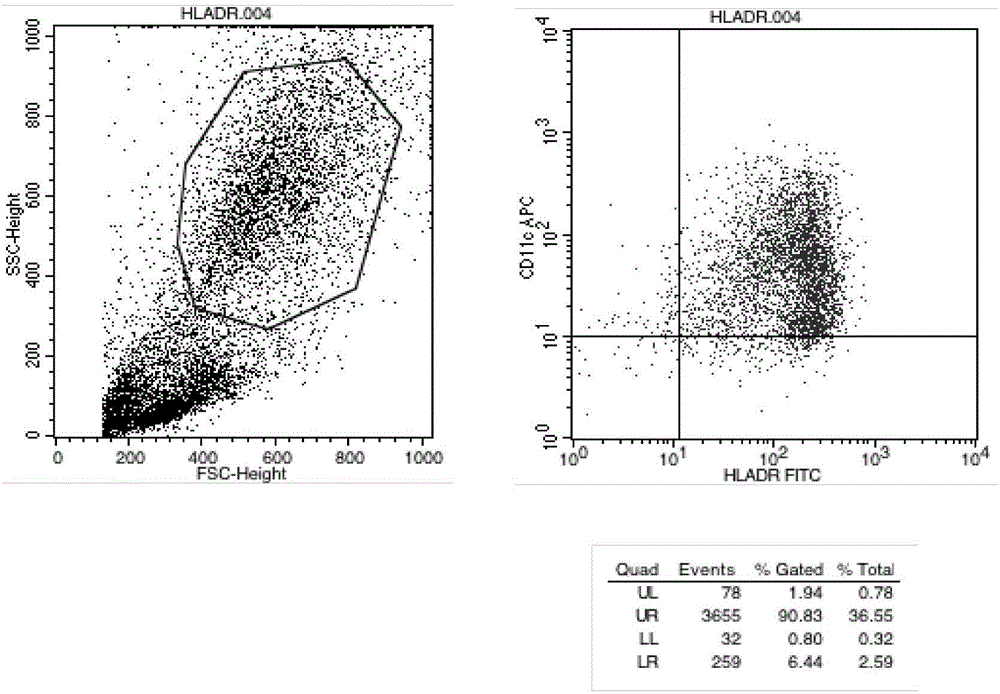
[0025] Example 2 Detection of Immunophenotype of DC Cells After Improving Maturation
[0026] Take DC cells after maturation (on the 6th day, mix the cells and take samples), wash them twice with PBS without calcium and magnesium, take 1×105 / mL each, and add them to the corresponding FCM tubes. Add 5 μl of monoclonal antibodies to be tested, including CD11C, HLA-DR, CD83, and CD86 antibodies, incubate at 4°C in the dark for 30 minutes, and shake once every 10 minutes to fully contact the cells with the antibodies. Washed twice with PBS, resuspended in 400 μl of PBS, and detected by flow cytometer FASCSCalibur (BD Biosciences), the results are shown in Table 1, Figure 2-4 .
[0027] Table 1
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com