Cancer vaccine composition
A cancer vaccine and composition technology, which can be used in vaccines, drug combinations, DNA/RNA vaccination, etc., and can solve problems such as unreported WT1 modified peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0301] Example 1 (Prediction of HLA Molecules Capable of Binding to WT1 Peptide)
[0302] able to work with WT1 187 The HLA molecules bound by the peptide (SEQ ID NO: 2) were predicted using the NetMHC2.0 server prediction program.
[0303] As a result, HLA-A*0201-restricted WT1 capable of inducing WT1-specific CTLs 187 Peptides, in terms of binding affinity to HLA-A*0206 molecules, were predicted in the NetMHC2.0 server ( http: / / www.cbs.dtu.dk / services / NetMHC-2.0 / ) is sorted higher.
Embodiment 2
[0304] Example 2 (preparation of WT1 from PBMCs of HLA-A*0206 positive healthy blood donors 187 Peptide-specific CTLs, and cytotoxicity assays of CTLs)
[0305] (1) Separation of PBMCs from HLA-A*0206 positive healthy blood donors, and preparation of DCs
[0306] First, PBMCs were isolated from the peripheral blood of each HLA-A*0206 healthy blood donor (three individuals) by Ficoll-Hypaque density gradient centrifugation. Then, CD14-positive cells were selected from the PBMCs using anti-human CD14 magnetic beads-DM (manufactured by Becton, Dickinson and company (BD)). In this case, it is considered that a large number of CD14-positive cells are present in the monocyte population. The selected CD14-positive cells were cultured in X-VIVO15 medium (manufactured by BioWhittaker, Walkersville, MD), supplemented with 1v / v% human AB serum, 800IU / mL GM-CSF (manufactured by Pepro Tech INC, Rocky Hill, NJ) and 1000 IU / mL IL-4 (manufactured by Pepro Tech INC), to prepare DCs.
[030...
Embodiment 3
[0332] Example 3 (verification limit WT1 187 HLA type of peptide-specific CTLs)
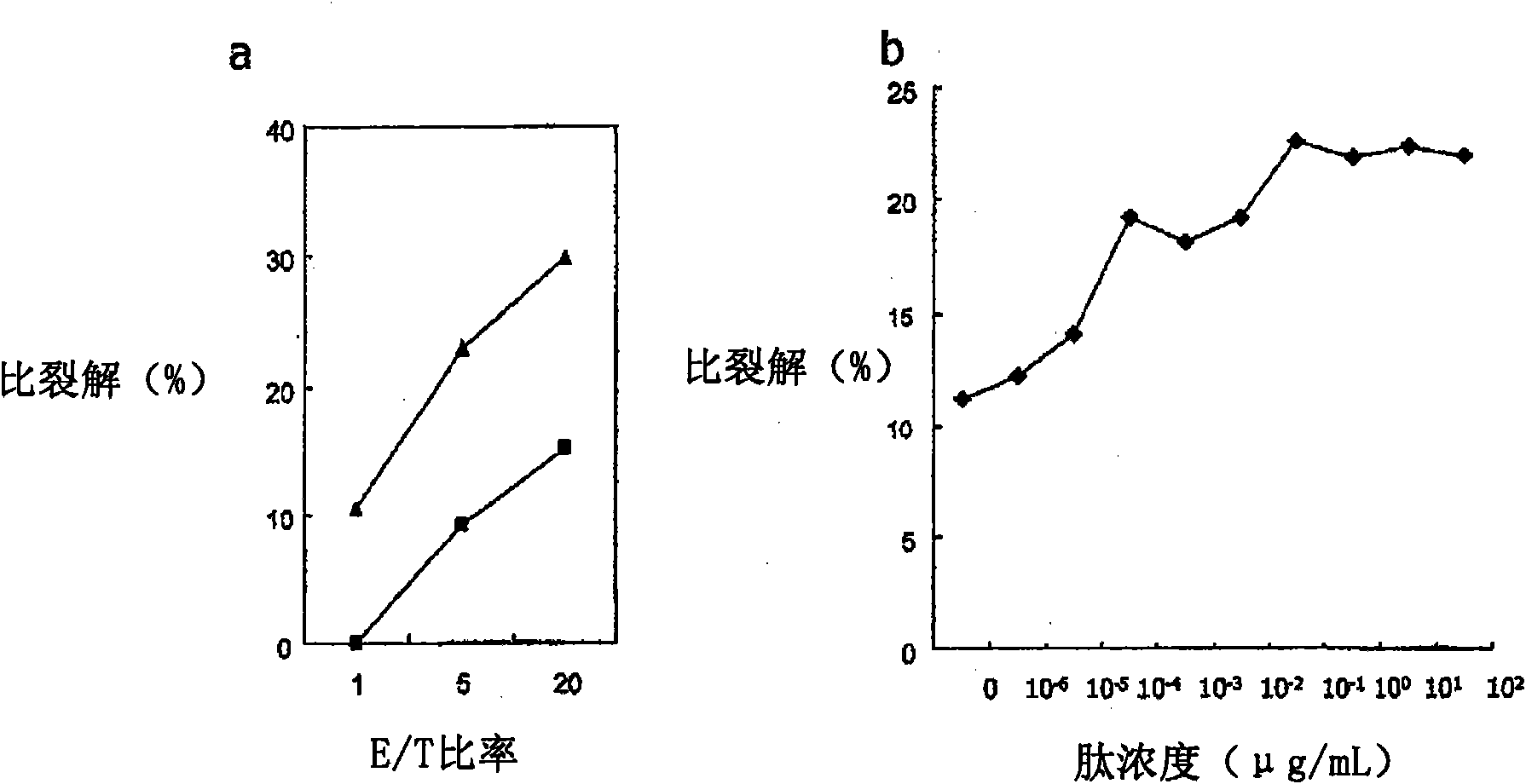
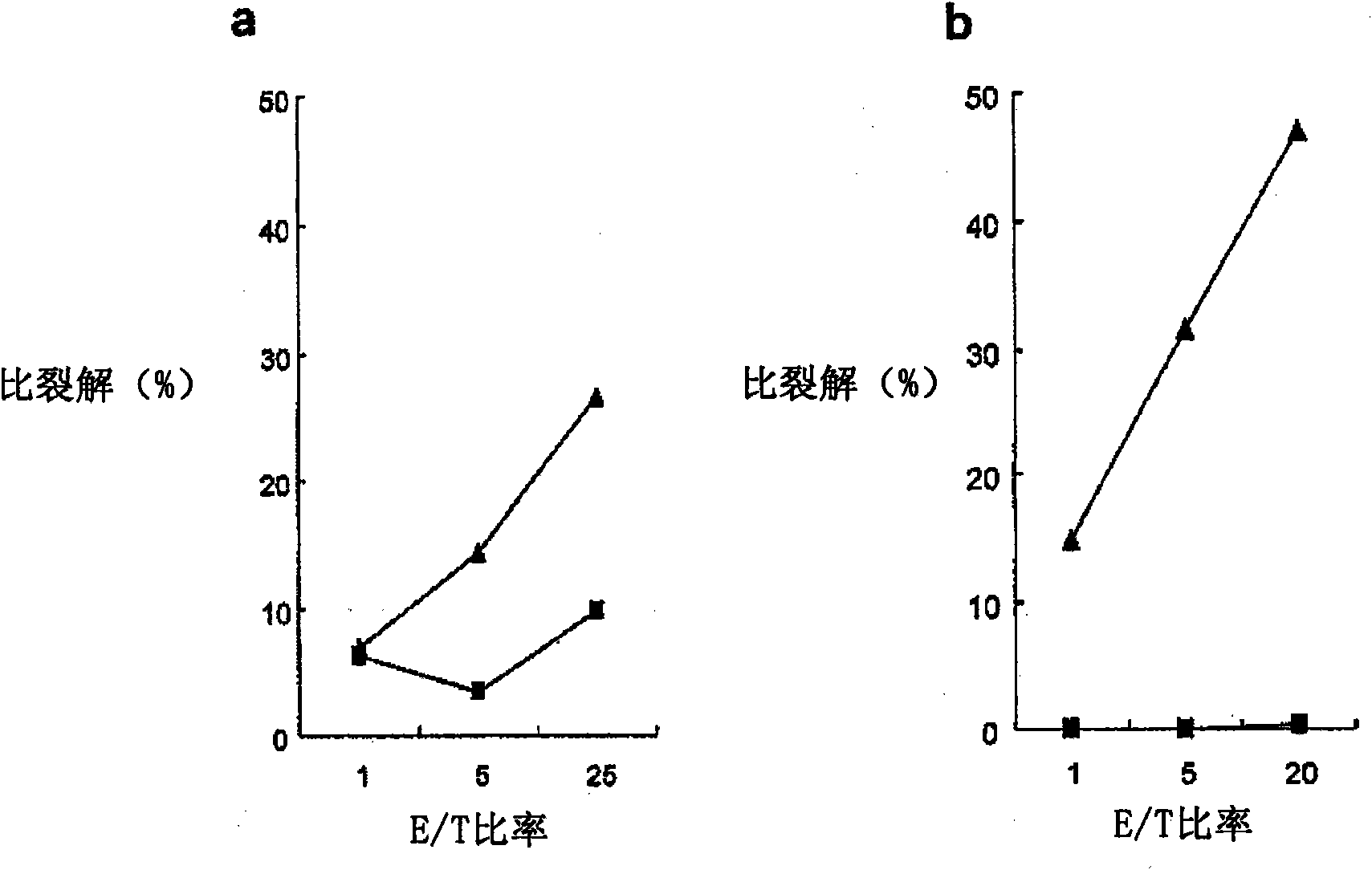
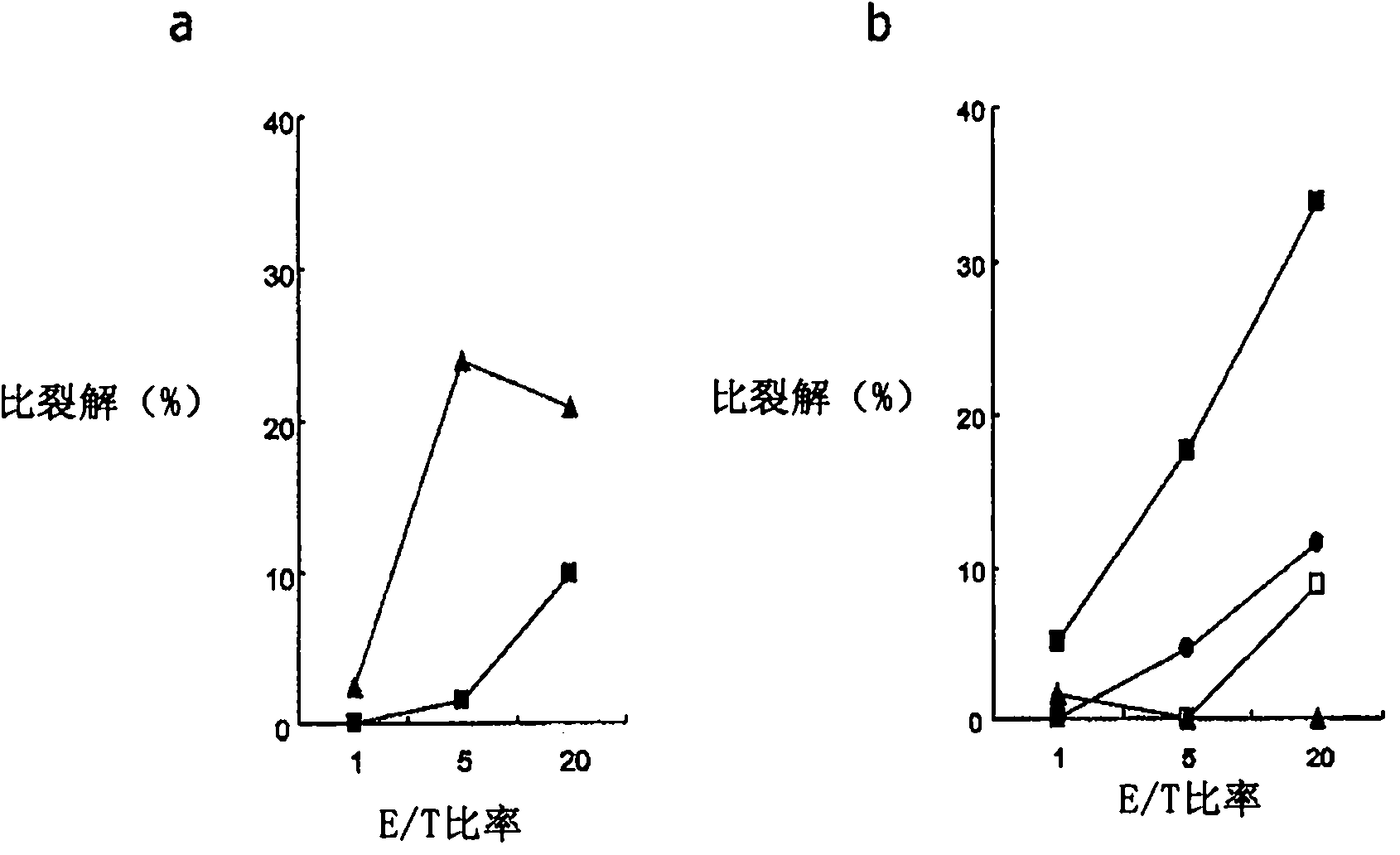
[0333] WT1 obtained in Example 2 was examined 187 Is the cytotoxic activity of peptide-specific CTLs restricted by class I HLA. performed in the presence or absence of mAbs against HLA class I or HLA class II 51 Cr release cytotoxicity test. Autologous B-LCLs were used as target cells. In this experiment, the E / T ratio was 5:1.
[0334] The results are shown in Figure 4 middle. Figure 4 a shows the use of B-LCLs (not expressing WT1 187 , HLA-A*0206 positive) as target cells, the results of experiments performed in the absence of mAbs against class I HLA (anti-HLA class I mAbs) and mAbs against HLA class II (anti-HLA class II mAbs) . Figure 4 b shows the use of WT1 187 Peptide-pulsed B-LCLs (expressing WT1 187 , HLA-A*0206 positive) as target cells, the results of experiments performed in the absence of anti-HLA class I mAbs and in the presence of anti-HLA class II mAbs. Figure 4 c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com