Artificial T helper cell epitopes as immune stimulators for synthetic peptide immunogens including immunogenic LHRH peptides
A technology of peptide immunogen and immunostimulatory sequence, which is applied in the field of conventional immunostimulatory sequence, and can solve the problems of uneven size and composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0082] The peptide immunogen of the present invention can be prepared by chemical methods known to those skilled in the art. See, for example, Fields et al., "Synthetic Peptides: User's Manual" Chapter 3, Grant, W.H. Ed., Freeman & Co., New York, NY, 1992, p.77. The peptide can be automatically matched Merrifield solid-phase peptide synthesis method, using t-Boc or Fmoc to protect α-NH 2 Or side chain amino acids, to synthesize. The equipment for peptide synthesis is provided commercially. An example is the 430A or 431 applied biological system peptide synthesizer.
[0083] After completing the assembly of the required peptide immunogen, the resin is processed according to standard procedures to cleave the peptide from the resin and deblock the functional groups on the amino acid side chains. The free peptide is purified by HPLC, and biochemical characteristics are identified by, for example, amino acid analysis, by sequencing, or by mass spectrometry. Methods of purifying and ide...
Embodiment 1
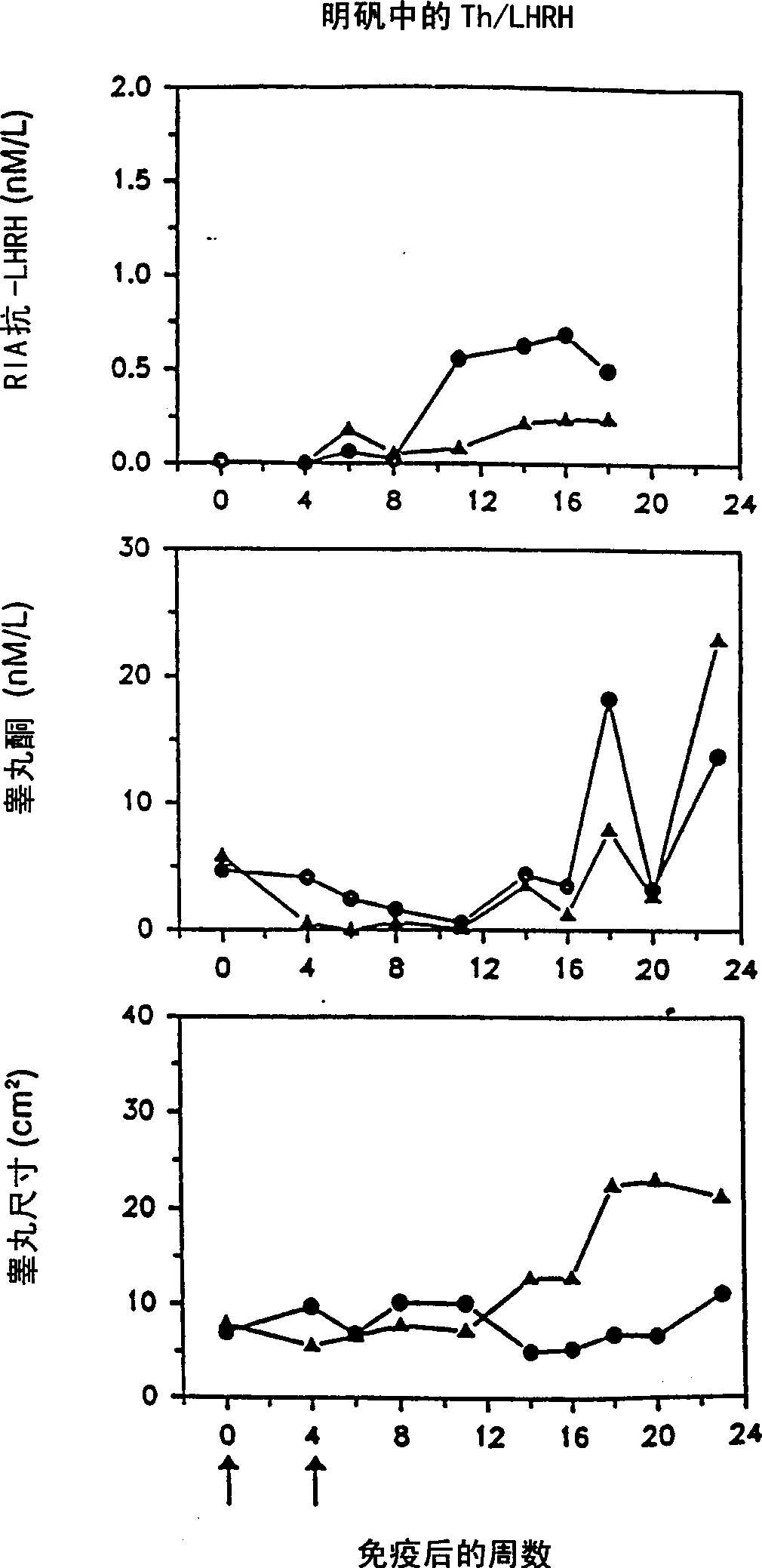
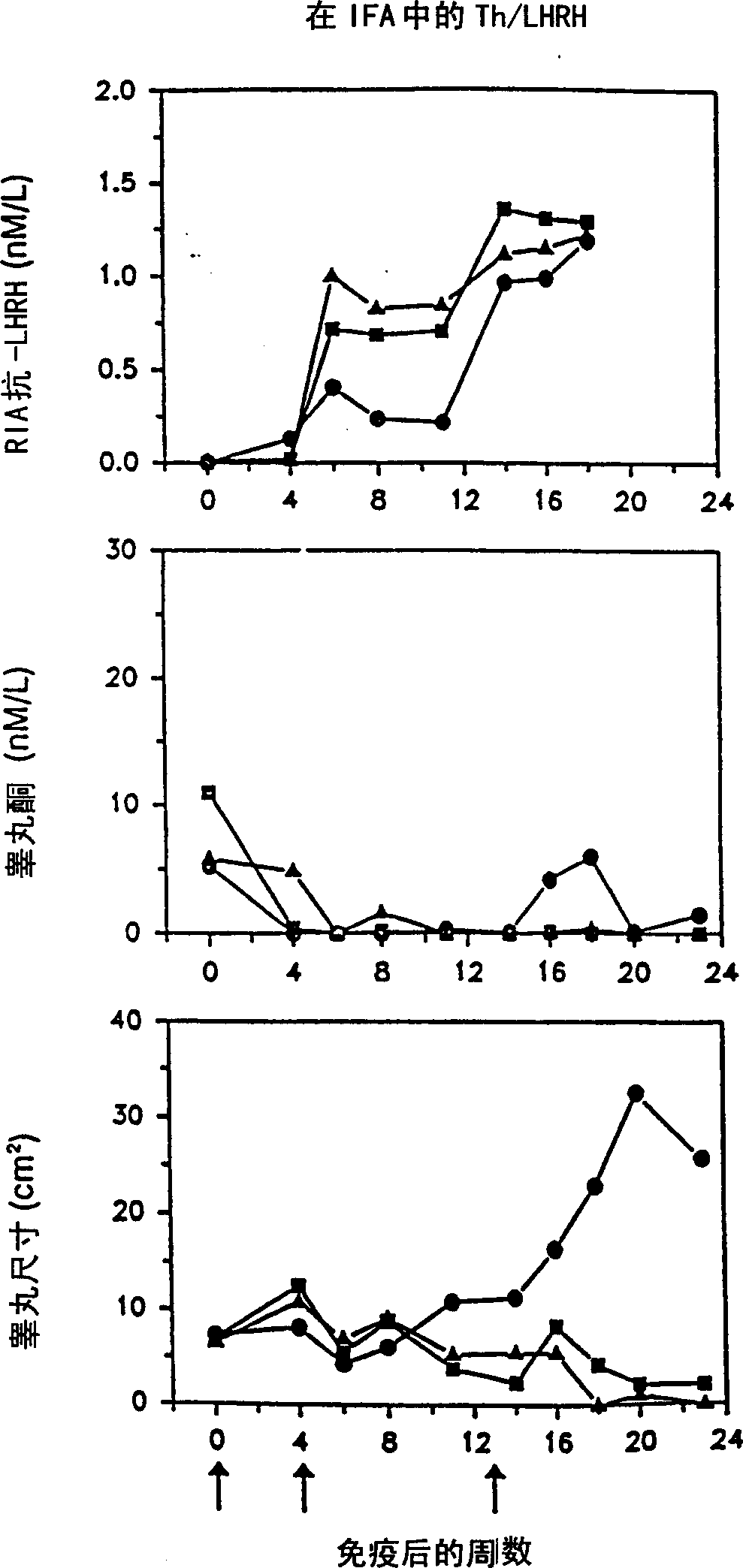
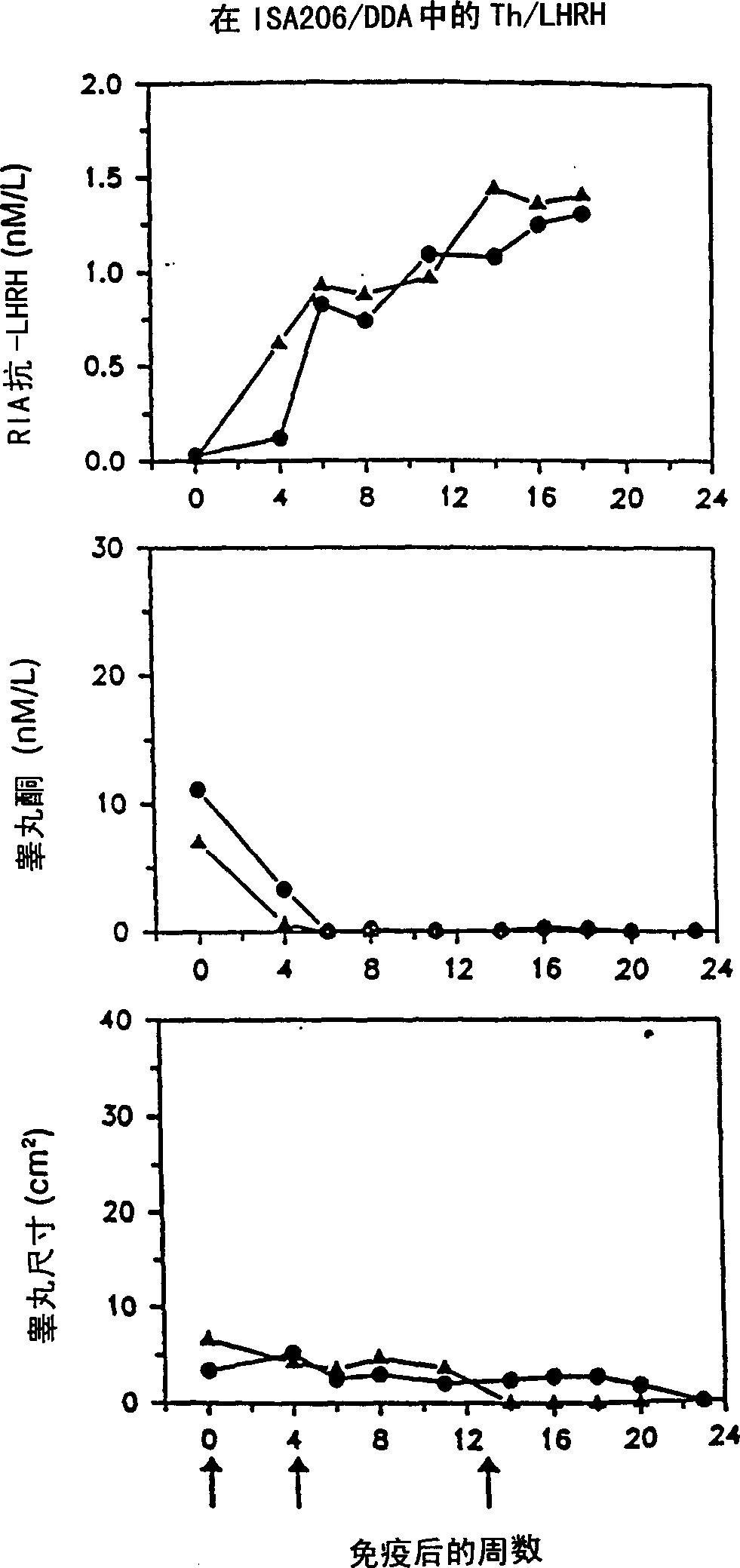
[0103] Immunize rats with peptide immunogen containing LHRH
[0104] The peptides listed in Tables 2a and 2b were synthesized and tested as described below.
[0105] A. Synthesis of peptides. Using Merrifield solid-phase synthesis technology, the peptides listed in Table 2a and 2b were synthesized using Fmoc chemicals on Applied Biosystems' automatic matching peptide synthesizer (Models 430, 431 and 433A). A peptide construct containing the constructed synthetic antigen library (SSAL), for example, the artificial Th site designated as SEQ ID NO: 3, is prepared by providing a mixture of desired amino acids selected for a specific position. After completing the assembly of the required peptide or combination of peptides, the resin is treated with trifluoroacetic acid according to standard procedures to remove the peptide from the resin and deblock the protective groups on the amino acid side chains.
[0106] The excised, extracted and cleaned peptides were purified by HP...
Embodiment 2
[0127] A mixture of LHRH peptides that induces more extensive immune castration in rats
[0128] It was confirmed that the relative effects of the various artificial TH epitopes / LHRH constructs shown in Example 1 can select a more effective peptide mixture that is combined into an enhanced immunogenicity. The Th / LHRH peptide immunogen mixture is more effective than any individual peptide in the mixture of US 5795551. Moreover, a mixture of individual constructs that carry promiscuous Th epitopes derived from MVF Th (SEQ ID NO: 2) and HbsAg Th (SEQ ID NOS: 18-20) provides a greater number of genetically diverse populations than those derived from only one The peptide composition of Th epitope mixed with Th epitope responds more widely. Therefore, a peptide composition containing the peptide mixture of the present invention derived from MVF Th and HbsAg Th was assembled, and the effect of the mixture was tested and compared with the composition containing the individual peptide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com