Medicine for treating and/or preventing cancer and application
A drug and cancer technology, applied in drug combinations, medical preparations containing active ingredients, anti-tumor drugs, etc., can solve problems such as failure, prolong patient survival, and fail to improve the clinical efficacy of PD-1 monotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Materials
[0037] Experimental reagents: The recombinant MUC1-MBP fusion protein vaccine was prepared according to the sequence shown in SEQ ID NO.1 by conventional vaccine preparation methods. Anti-PD-1 antibody RMP1-14 and rat IgG 2a isotype control antibody were purchased from BioXcell. Sterile water for injection and normal saline were purchased from Sichuan Kelun Pharmaceutical Co., Ltd.
[0038] Experimental animals: C57 BL / 6 mice (SPF grade, half male and half male, 6-8 weeks old, body weight 18-22 g) were purchased from Shenyang Changsheng Biotechnology Co., Ltd.
[0039] 2. Method
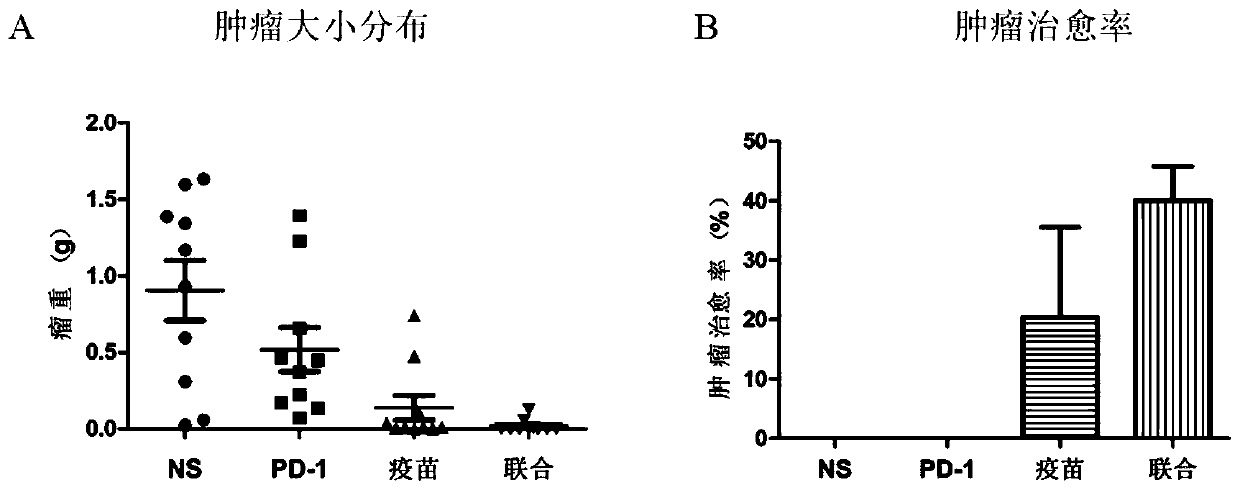
[0040] 2.1 Immunization of mice
[0041] The mice were weighed and randomly divided into groups of 10 mice each, including normal saline control group (NS group), immune checkpoint inhibitor anti-PD-1 antibody injection group (PD-1 group), and recombinant MUC-MBP fusion group. Protein vaccine group (vaccine group), recombinant MUC-MBP fusion protein vaccine preparation combin...
Embodiment 2
[0052] 1. Materials
[0053] Experimental reagents: The recombinant MUC1-MBP fusion protein vaccine was prepared according to the sequence shown in SEQ ID NO.1 by conventional vaccine preparation methods. Anti-PD-1 antibody RMP1-14 and ratIgG2a isotype control antibody were purchased from BioXcell. Sterile water for injection and normal saline were purchased from Sichuan Kelun Pharmaceutical Co., Ltd. Experimental animals: C57BL / 6 mice (SPF grade, half male and half male, 6-8 weeks old, weighing 18-22 g) were purchased from Shenyang Changsheng Biotechnology Co., Ltd.
[0054] 2. Method
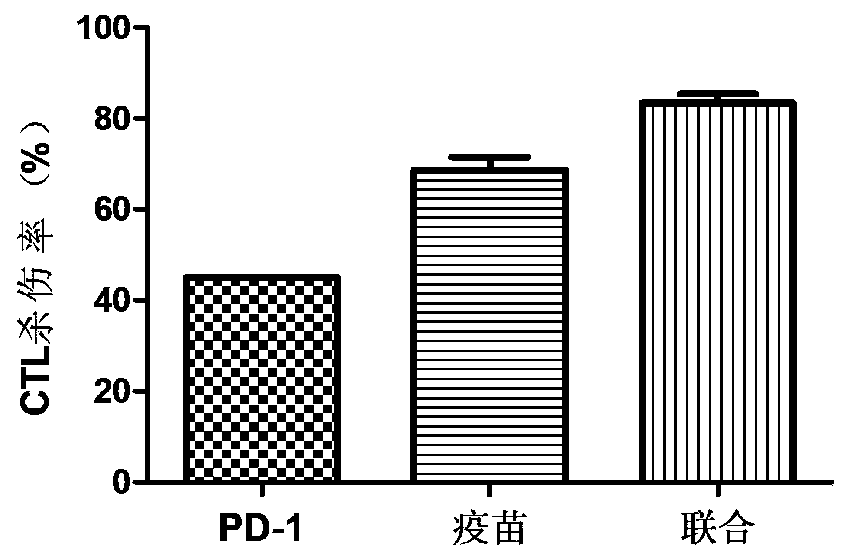
[0055] 2.1 Effect of recombinant MUC1-MBP fusion protein vaccine combined with anti-PD-1 antibody on mouse CTL killing activity
[0056] CTL killing is the gold standard for evaluating antitumor effects. In order to verify whether recombinant MUC1-MBP fusion protein vaccine combined with PD-1 antibody treatment can induce stable MUC1-specific CTL killing in mice. As mentioned above, on the...
Embodiment 3
[0062] 1. Materials
[0063] Experimental reagents: The recombinant MUC1-MBP fusion protein vaccine was prepared according to the sequence shown in SEQ ID NO.1 by conventional vaccine preparation methods. Anti-PD-1 antibody RMP1-14 and rat IgG2a isotype control antibody were purchased from BioXcell. Sterile water for injection and normal saline were purchased from Sichuan Kelun Pharmaceutical Co., Ltd. MUC1 polypeptide was synthesized by Shanghai Ziyu Biotechnology Co., Ltd., goat anti-mouse IgG2c-HRP and rat anti-mouse IgG1-HRP were purchased from SouthernBiotech, and goat anti-mouse IgG (H+L) was purchased from Proteintech. Experimental animals: C57 BL / 6 mice (SPF grade, half male and half male, 6-8 weeks old, body weight 18-22 g) were purchased from Shenyang Changsheng Biotechnology Co., Ltd.
[0064] 2. Method
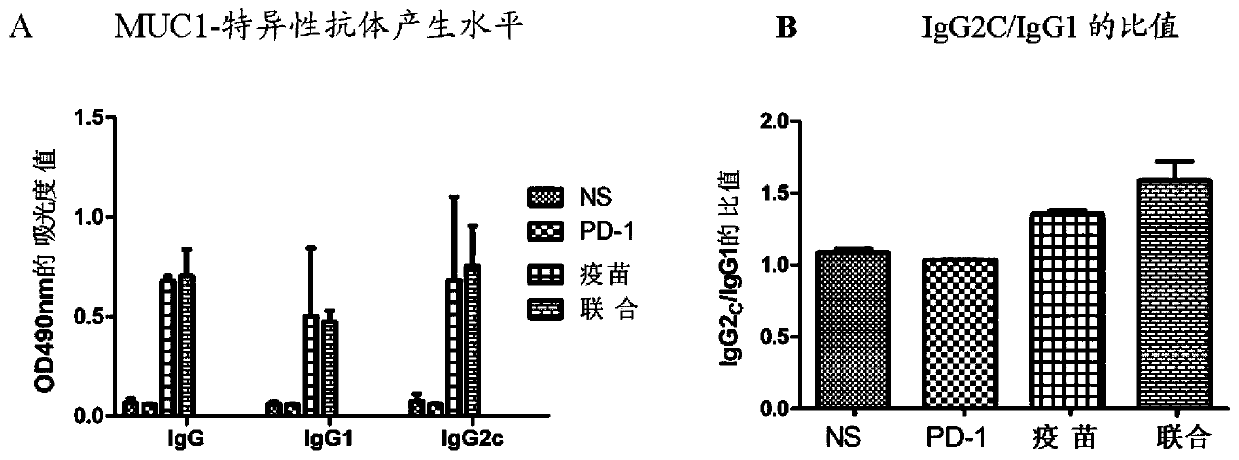
[0065] 2.1 Effect of recombinant MUC1-MBP fusion protein vaccine combined with anti-PD-1 antibody on the production of MUC1-specific antibodies and subclasses
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com