Application of recombinant attenuated listeria in preparation of mesothelin high-expression cancer therapeutic vaccine
A technology for Listeria and cancer treatment, applied in recombinant DNA technology, bacteria, bacterial peptides, etc., can solve problems such as defects and low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1. Preparation of vaccine bacteria LiΔactAplcB-Meso
[0102] (1) Synthesis of Mesothelin Gene Fragment
[0103] Query the mesothelin protein gene from NCBI, then optimize it according to the codons of Listeria monocytogenes, add a HindⅢ enzyme cleavage site upstream, add an XhoI enzyme cleavage site downstream, and finally obtain the DNA sequence directly by synthesis (provided by the company The cloning plasmid pUC57-Meso was obtained), (the sequence is shown in Sequence 1 of the Sequence Listing).
[0104] (2) Construction of targeting plasmid
[0105] ① Construction of intermediate plasmid pCW203-Meso
[0106] Plasmids pUC57-Meso and pCW203 were extracted according to the instructions of the plasmids, and eluted with 30 μL Elution Buffer.
[0107] pUC57-Meso and pCW203 were double-digested with HindⅢ and XhoⅠ, 20 μL of enzyme digestion system: pUC57-Meso or pCW203 2 O Make up the system to 20 μL. Digest the plasmid in a water bath at 37°C for 1 hour, add ...
Embodiment 2
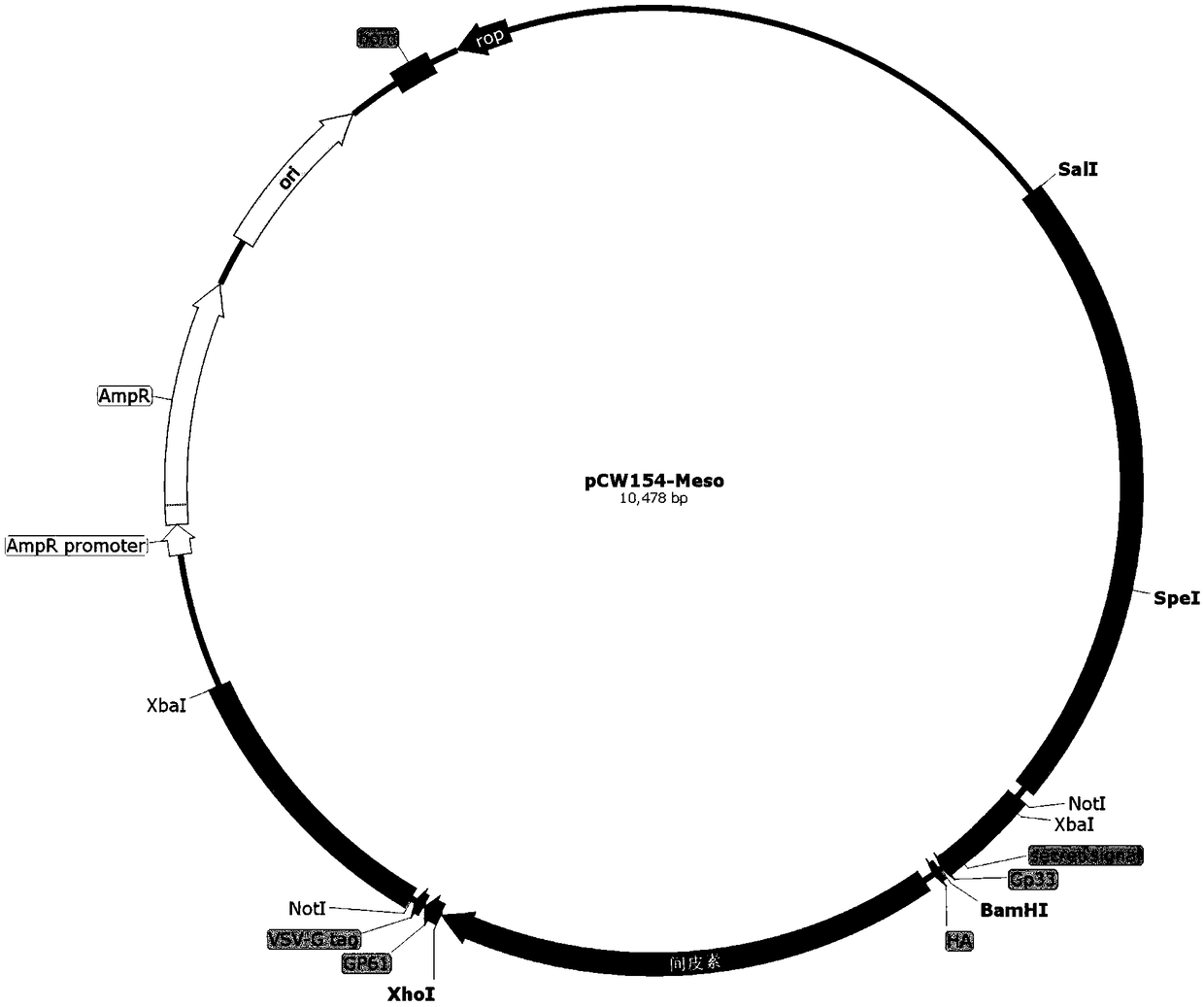
[0144] Embodiment 2: Preparation of vaccine bacteria LmΔactAplcB-Meso
[0145] (1) Synthesis of Mesothelin Gene Fragment
[0146] With embodiment 1 (1).
[0147] (2) Construction of targeting plasmid
[0148] ①The construction of the intermediate plasmid pCW203-Meso is the same as in Example 1(2)①
[0149] ②Construction of targeting plasmid
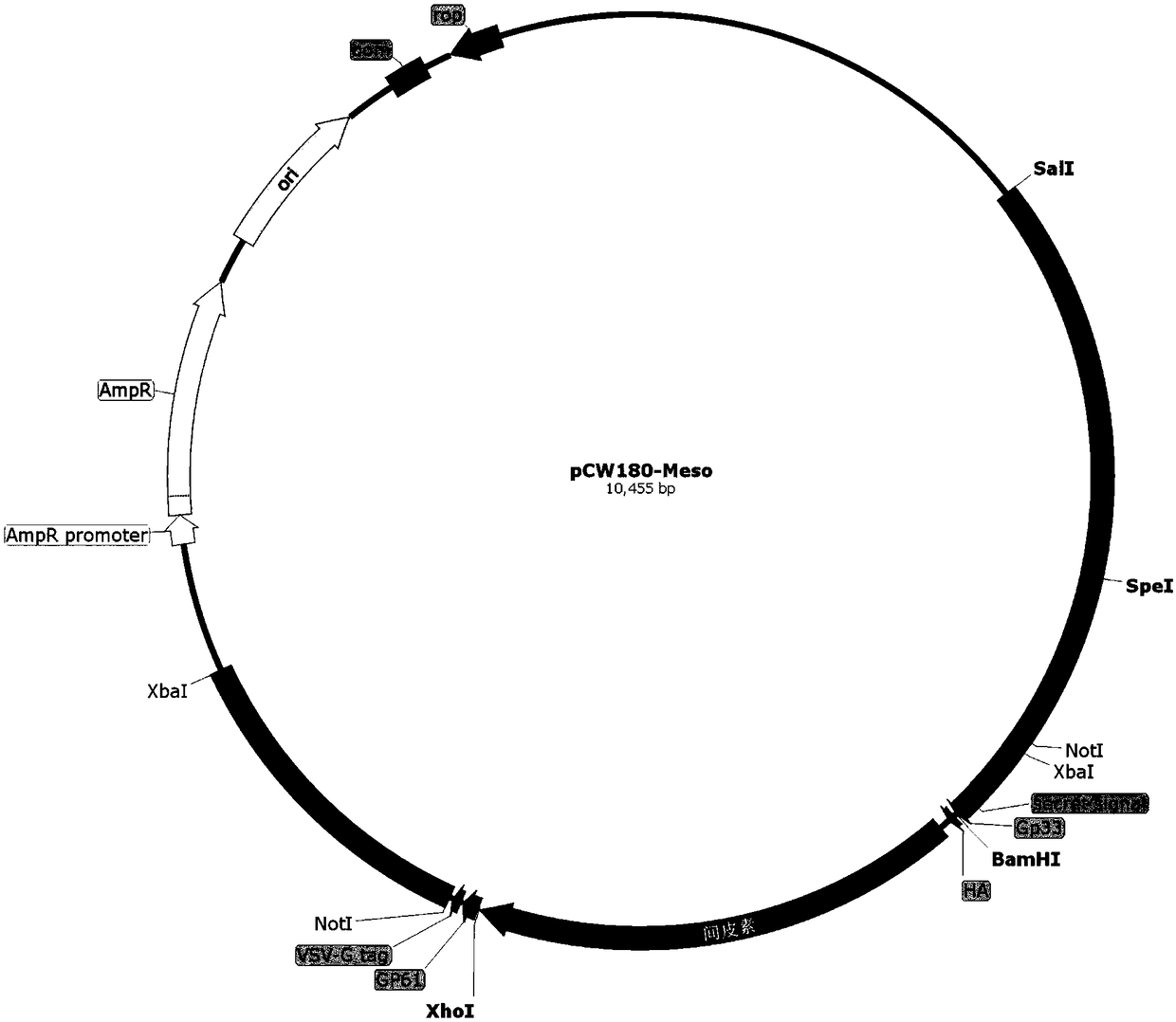
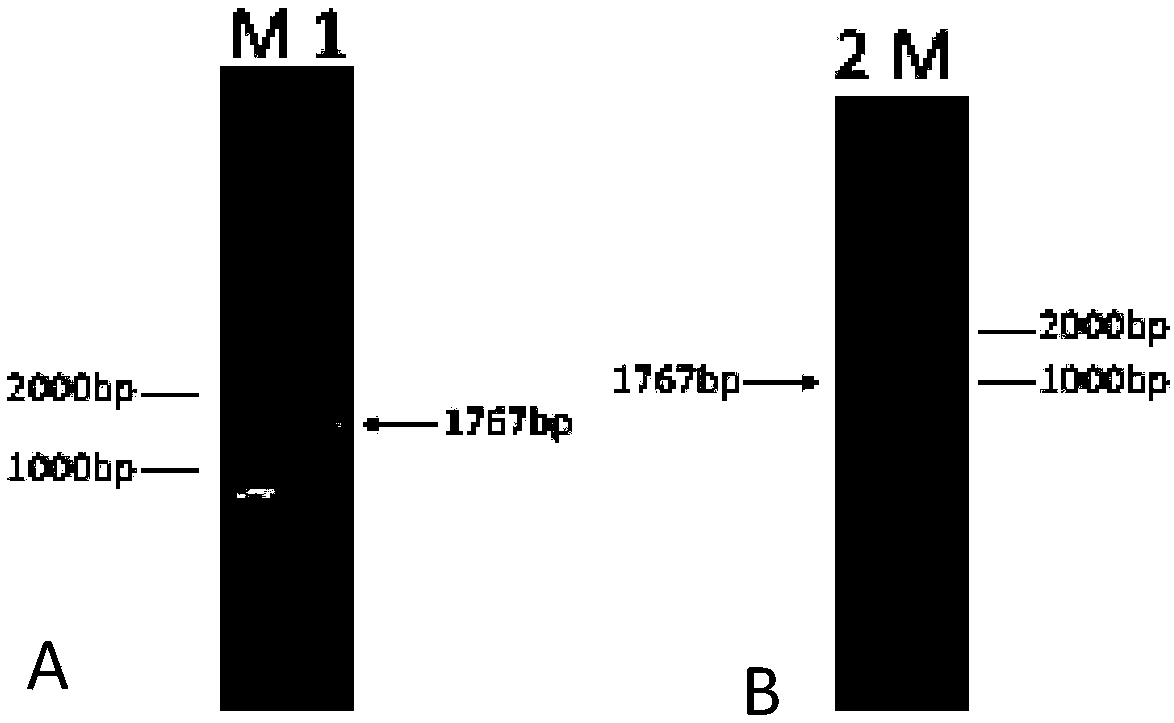
[0150] Plasmid pCW180 was extracted and eluted with 30 μL Elution Buffer. Mix plasmid pCW180 and intermediate plasmid pCW203-Meso with restriction endonucleases BamHI and XhoI respectively according to the system of (2)① (replace HindIII enzyme with BamHI enzyme), carry out enzyme digestion and dephosphorylation, and gel recovery after electrophoresis The small fragment after pCW203-Meso digestion is the insert fragment (1840bp) and the pCW180 vector backbone (long fragment after digestion, about 8621bp in length). According to the system of (2) ①, mix and connect and transform into E. coli DH5α, spread on LA plate, perform PCR scree...
Embodiment 3
[0167] Example 3: Evaluation of the therapeutic effect of vaccine strains on cancer with high expression of mesothelin
[0168] (1) CT26-MESO tumor cell culture and tumor model establishment
[0169] Use RPMI1640 Medium containing 10% fetal bovine serum, 1% penicillin, and streptomycin at 37°C in 5% CO 2 Cultivate CT26-MESO cells in an incubator until the density reaches about 80%, rinse the cells with Hank’s solution for 2 to 3 times, digest with trypsin and resuspend with PBS for counting, and prepare the cells to a concentration of 5×10 6 / ml of cell suspension, 6-8 weeks old BALB / c female mice were injected with 100 μl of cell suspension through the tail vein to establish a mouse lung metastatic tumor model and observe the survival rate.
[0170] (2) Comparing homologous and heterologous priming-boosting immunotherapy on model mice.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com