Vector containing double-target chimeric antigen receptor gene, CAR-T cell and application of CAR-T cell
A chimeric antigen receptor and dual-target technology, which is applied in the field of tumor cell immunotherapy, can solve the problem of less gastric cancer and achieve the effects of strong proliferation, reduced economic pressure, and long-lasting vitality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1. Dual-target chimeric antigen receptor and its coding gene for treating gastric cancer
[0027] This example provides a dual-target chimeric antigen receptor for the treatment of gastric cancer. The specific splicing method is as follows: sequentially splicing signal peptides from the N-terminal to the C-terminal, and can respectively recognize CD44 and EpCAM single chains on the surface of gastric cancer stem cells Antibody ScFv, strepII, CD8hinge, leukocyte antigen differentiation group molecule transmembrane region CD28-TM+ICD, 4-1BB, ζ chain of CD3 (leukocyte antigen differentiation group molecule 3), F2A peptide, IL-7, F2A peptide and CCL19, Finally, a complete chimeric antigen receptor (CAR) molecule capable of treating gastric cancer was obtained. Each of the above structural fragments can perform the following functions: signal peptide can secrete CAR extracellularly; CD44 single-chain antibody ScFv specifically recognizes CD44 protein on the surface o...
Embodiment 2
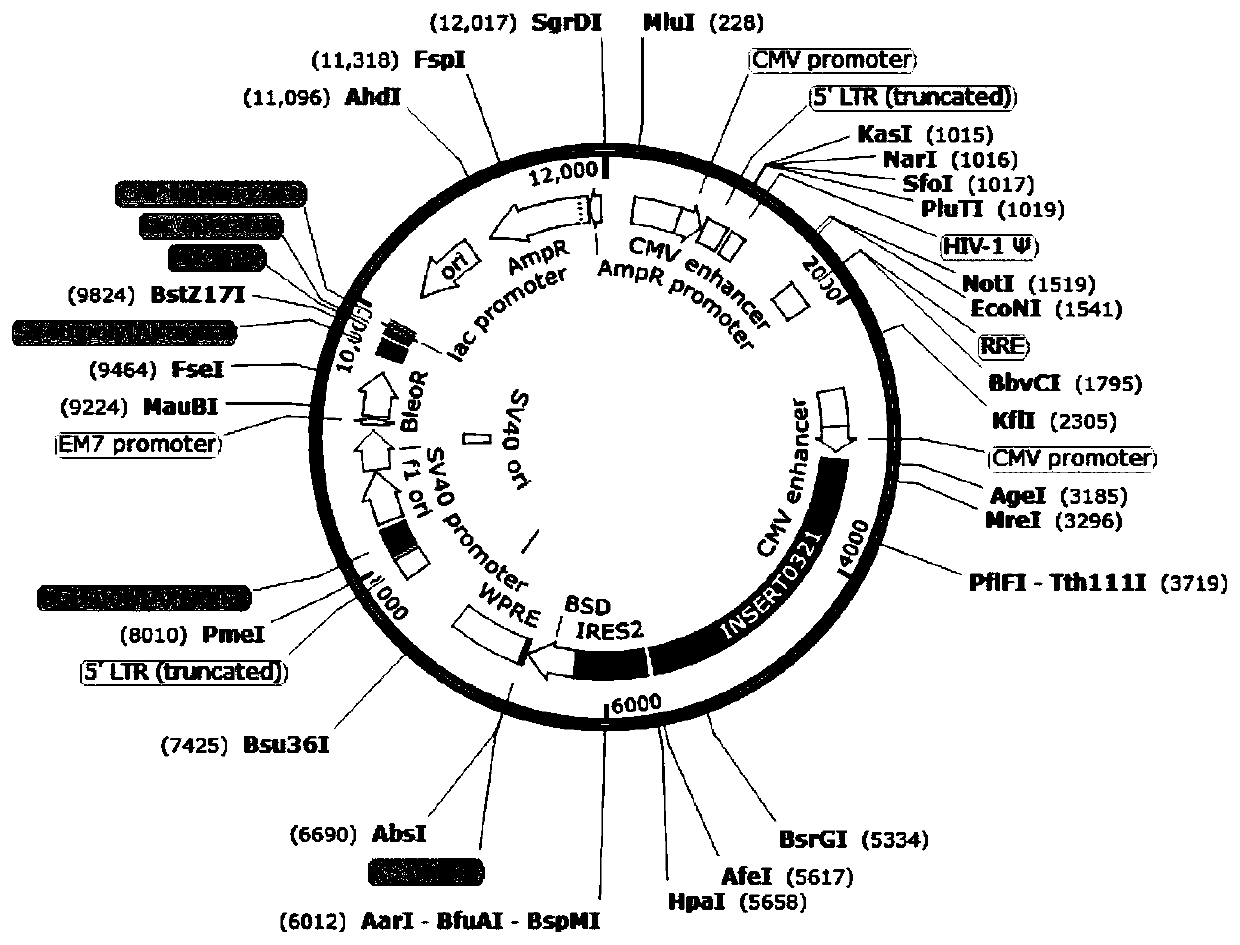
[0029] Example 2. Construction of a dual-target chimeric antigen receptor recombinant expression vector for the treatment of gastric cancer
[0030] This embodiment provides a dual-target chimeric antigen receptor recombinant expression vector and its construction method. The construction method of the vector specifically includes the following steps:
[0031] S1. Construction of recombinant expression plasmid pTK-881-CD44-EpCAM
[0032] (1) Preparation of experimental materials
[0033] ①Preparation method of LB liquid culture medium: Weigh 5g liquid medium dry powder into a 500mL conical flask with an electronic balance, add 100mL ultrapure water, seal it, sterilize it in a high-pressure steam sterilizer, and cool it to 40℃~50℃ Add ampicillin at a ratio of 1000:1, mix carefully, transfer to a clean 500mL reagent bottle for later use, and store at 4°C.
[0034] The preparation method of LB solid medium: Weigh 5g solid medium dry powder into a 500mL Erlenmeyer flask with an ...
Embodiment 3
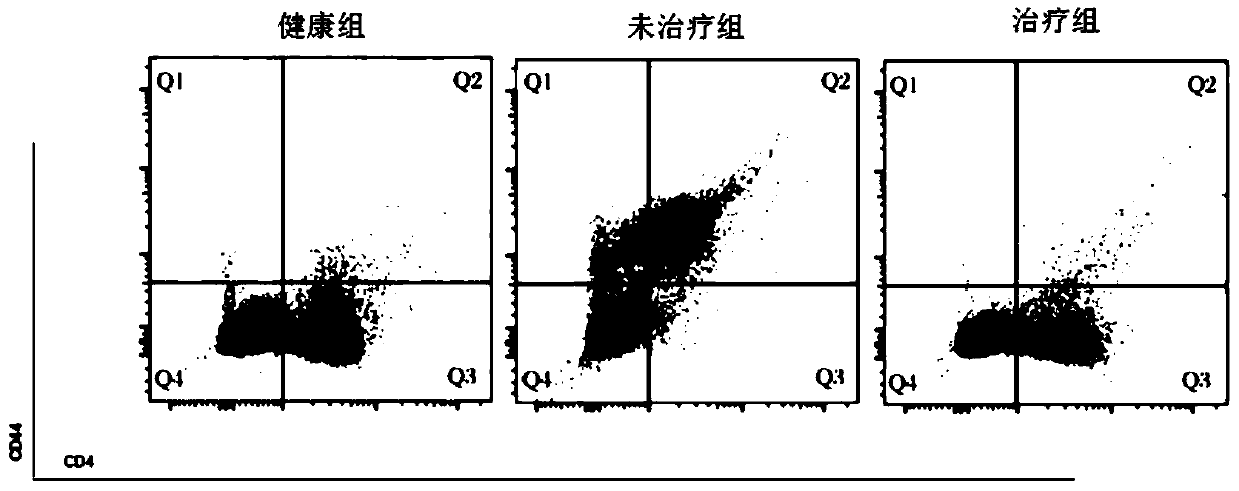
[0082] Example 3. Preparation of dual-target chimeric antigen receptor T cells (CAR-T) for the treatment of gastric cancer
[0083] S1, PBMC cell separation
[0084] Use a vacuum blood collection tube containing heparin to collect about 6mL of human peripheral fresh blood;
[0085] Dilution: add an equal volume of PBS at room temperature, and gently pipette to mix;
[0086] Adding samples: Take a 50mL centrifuge tube, draw 6mL Ficoll (lymphocyte separation solution) into the centrifuge tube (the volume ratio of Ficoll to blood before dilution is 1:1), tilt the tube at 45°, and put the diluted blood on the surface of Ficoll Slowly add to the top of Ficoll along the tube wall about 1cm above;
[0087] Centrifugation: Centrifuge at 2000rpm for 30min at 18-20°C. After centrifugation, it is divided into four layers from the bottom of the tube to the liquid surface, which are red blood cell and granulocyte layer, stratified liquid layer, mononuclear cell layer, and plasma layer; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com