Chimeric antigen receptor T cells and application thereof
A chimeric antigen receptor and cell technology, applied in the field of biomedicine, can solve the problems of inability to recruit peripheral blood immune cells into the tumor, and inability to secrete exogenous IL-7 protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The preparation of embodiment 1 lentiviral vector
[0057] Whole-gene synthesis of the following nucleic acid molecules:
[0058] ① A CAR molecule (SEQ ID NO: 2) formed by tandem nucleic acid sequences of GM-CSF signal peptide, MUC1 scFv, CD28 transmembrane domain, CD28 intracellular domain, CD3ζ, and TLR2;
[0059] ② A nucleic acid molecule formed in series by the nucleic acid sequences of IL-7 signal peptide (SEQ ID NO: 7) and IL-7 (without IL-7 signal peptide) (SEQ ID NO: 4);
[0060] ③ CCL19 nucleic acid molecule (SEQ ID NO: 6);
[0061] ④ A secreted IL-7 molecule formed by tandem nucleic acid sequences of IL-2 signal peptide (SEQ ID NO:3) and IL-7 (without IL-7 signal peptide) (SEQ ID NO:4);
[0062] ⑤ Regulatory CCL19 molecules formed in series by 4×NFAT-RE-minP promoter (SEQ ID NO:5) and CCL19 (SEQ ID NO:6);
[0063] SEQ ID NO: 7:
[0064] atgttccatgtttcttttaggtatatctttggacttcctcccctgatccttgttctgttgccagtagcatcatct.
[0065] The synthesized CAR molecule was c...
Embodiment 2
[0066] Embodiment 2 Preparation of recombinant lentivirus
[0067] (1) Cultivate 293T cells in a 10cm culture dish, the culture medium is DMEM high glucose medium+10%FBS (fetal bovine serum)+1% double antibody (100× penicillin-streptomycin mixed solution);
[0068] (2) When the 293T cell density in the culture dish reaches 80%, replace the medium with DMEM high glucose medium + 1% FBS + 1% double antibody;
[0069] (3) After replacing the medium and culturing for 2 hours, prepare a transfection reagent, take 500 μL opti-DMEM into a 15 mL centrifuge tube, add 7.2 μL of PEI (linear polyethyleneimine) with a concentration of 10 μg / μL, and mix slightly. Stand still for 5 minutes;
[0070] (4) Put 500 μL opti-DMEM into a 1.5 mL centrifuge tube, add the plasmid mixture shown in Table 1 into the centrifuge tube, mix well, add it to the transfection reagent, mix it upside down, and let it stand for 20 minutes;
[0071] Table 1
Embodiment 3
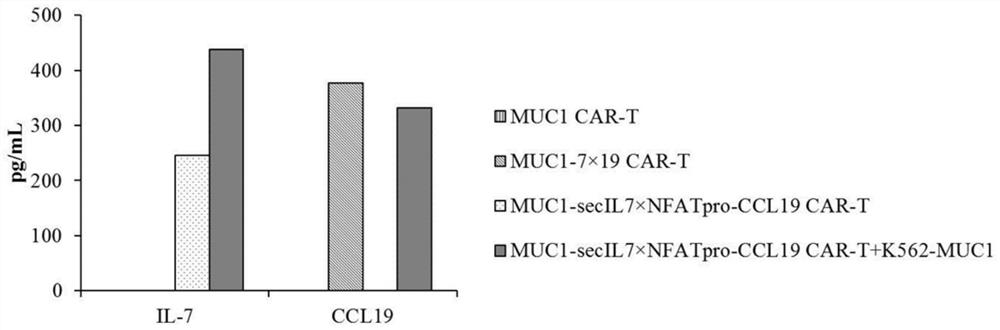
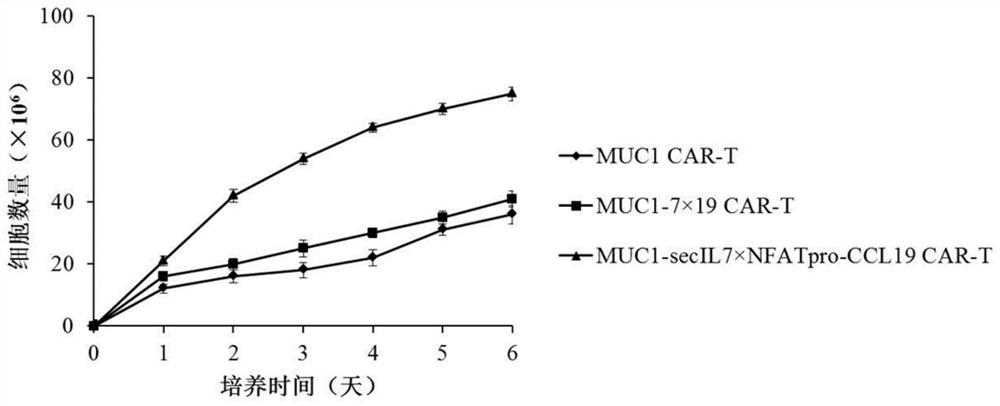
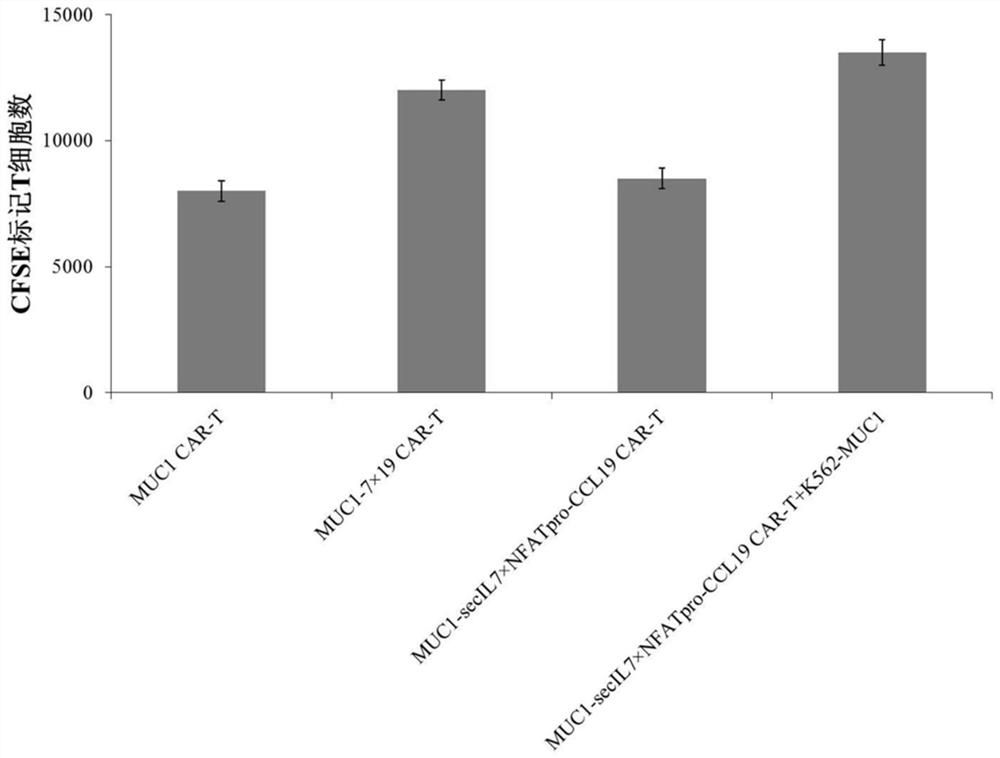
[0079] Example 3 Preparation of CAR-T cells expressing IL-7 and CCL19
[0080] (1) After sorting Pan T cells from umbilical cord blood, count the cells and adjust the concentration to 1×10 6 cells / mL, then add 10 μL of Miltenyi TransAct T cell reagent to each ml of cell suspension, and replace it with fresh medium (IMDM medium + 5% FBS (fetal bovine serum) + 1% double antibody ( 100×penicillin-streptomycin mixed solution)+IL-2);
[0081] (2) will 3×10 7 The activated T cells were centrifuged at 300g for 5min, and resuspended with 3mL medium (IMDM medium + 5% FBS (fetal bovine serum) + 1% double antibody (100 × penicillin-streptomycin mixed solution));
[0082] (3) Inoculate 3 mL of T cell suspension in a 6-well plate, 1 mL per well;
[0083] (4) Add the following recombinant lentivirus combinations to a 6-well plate, 9 mL per well;
[0084] ① Supernatant of recombinant lentivirus expressing CAR;
[0085] ② Equal amounts of recombinant lentivirus supernatant expressing CAR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com