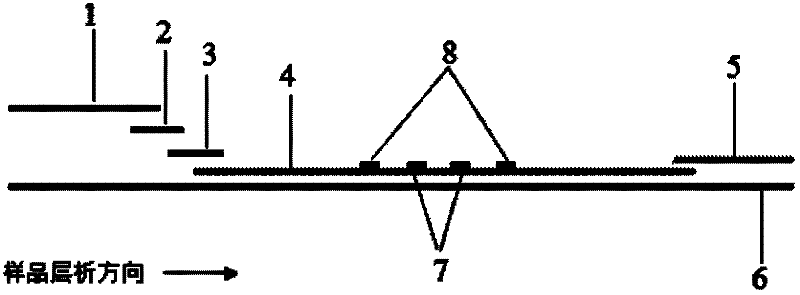
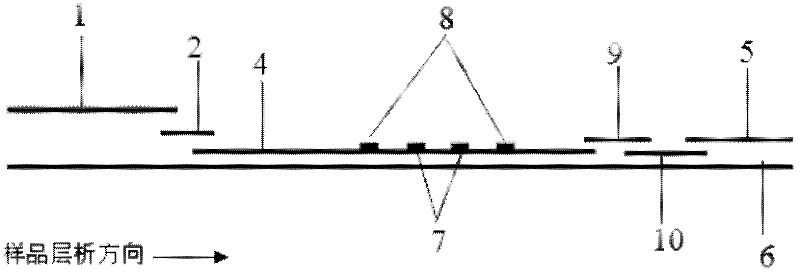
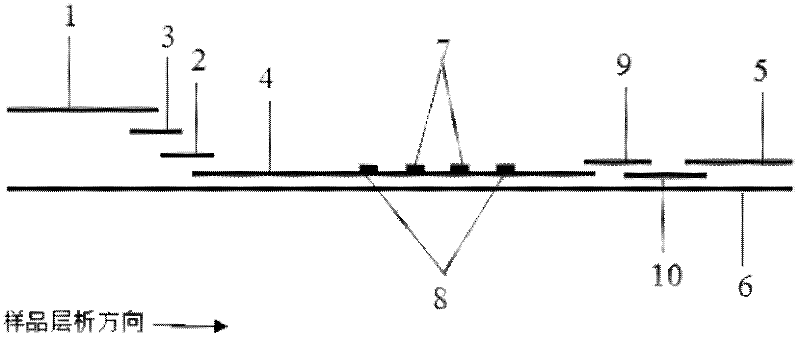
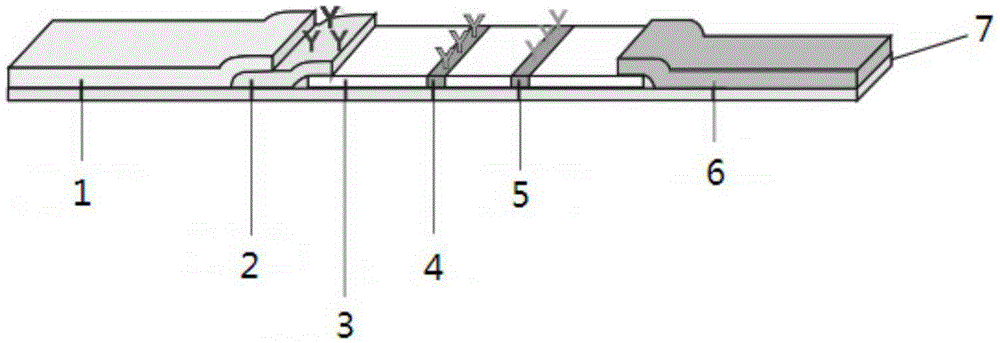
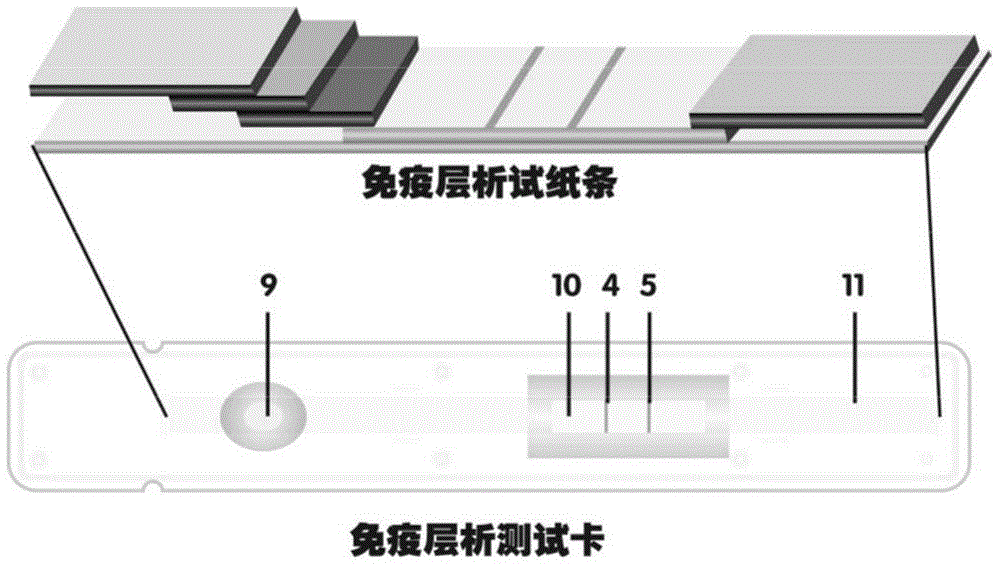
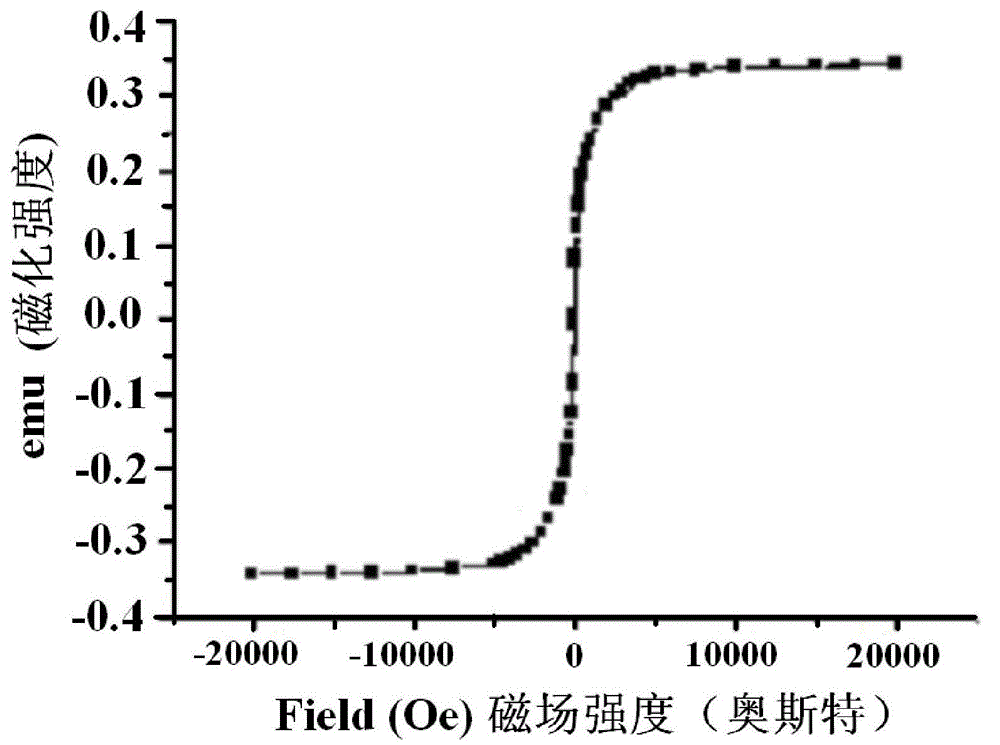
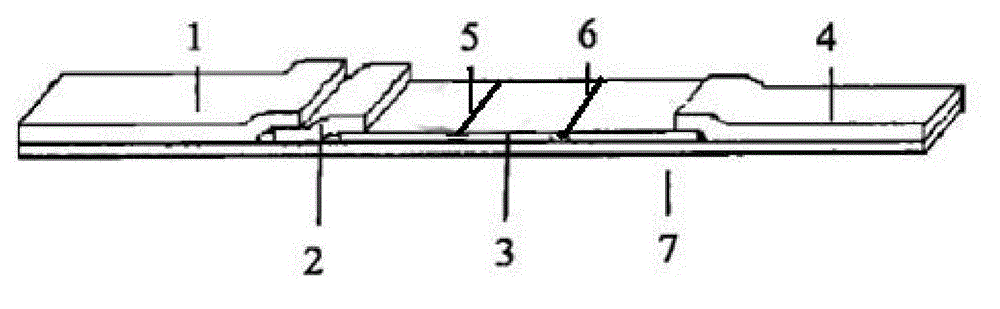
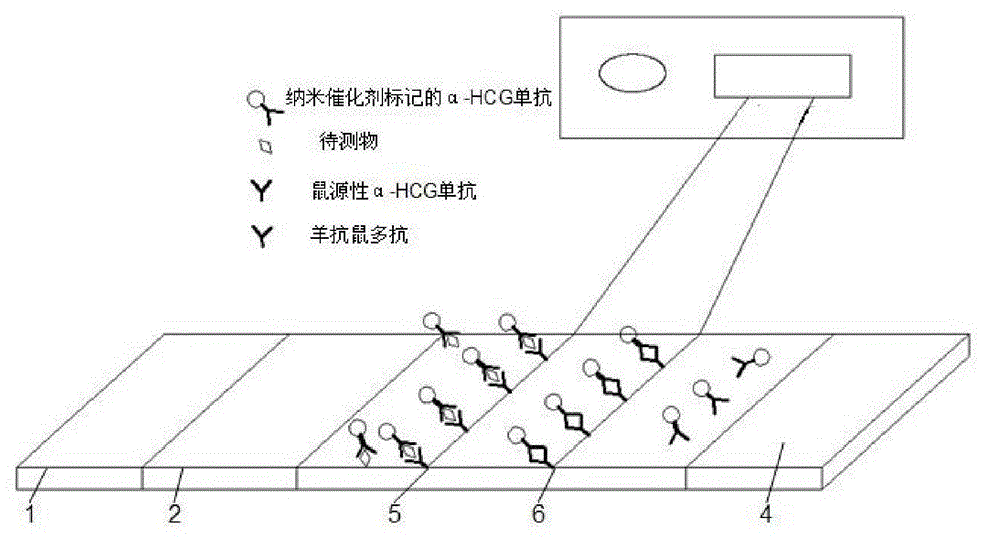
Patents
Literature
89results about How to "Reduce non-specificity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
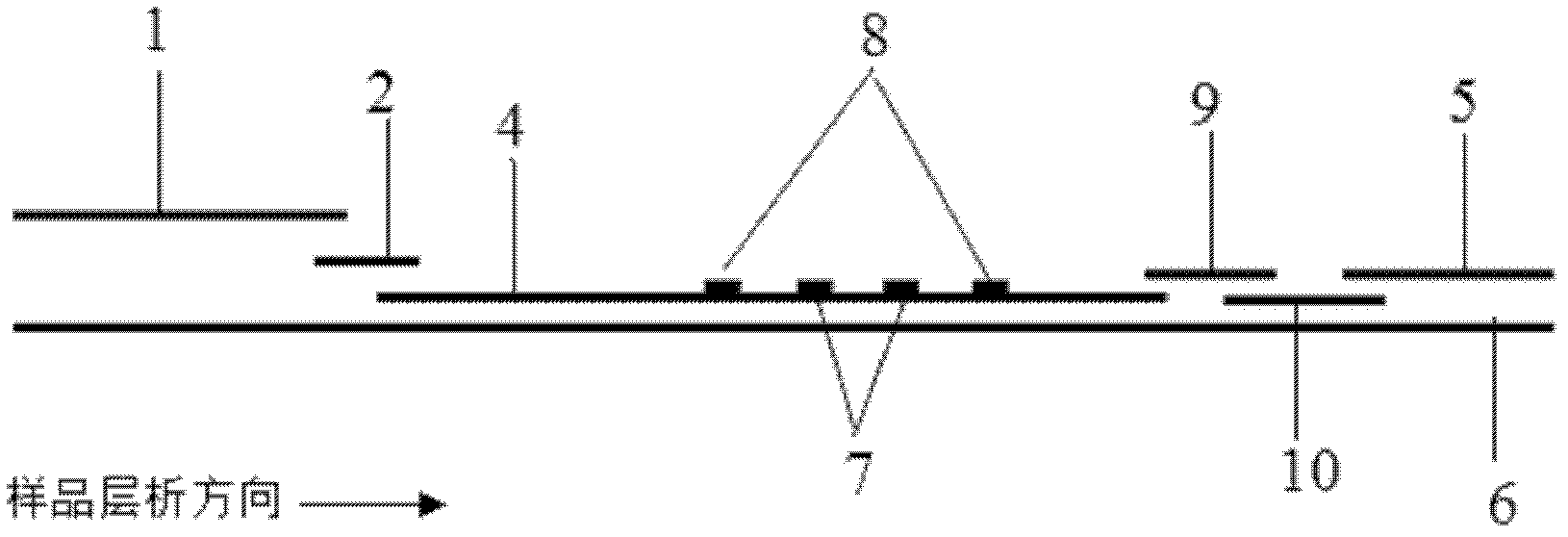
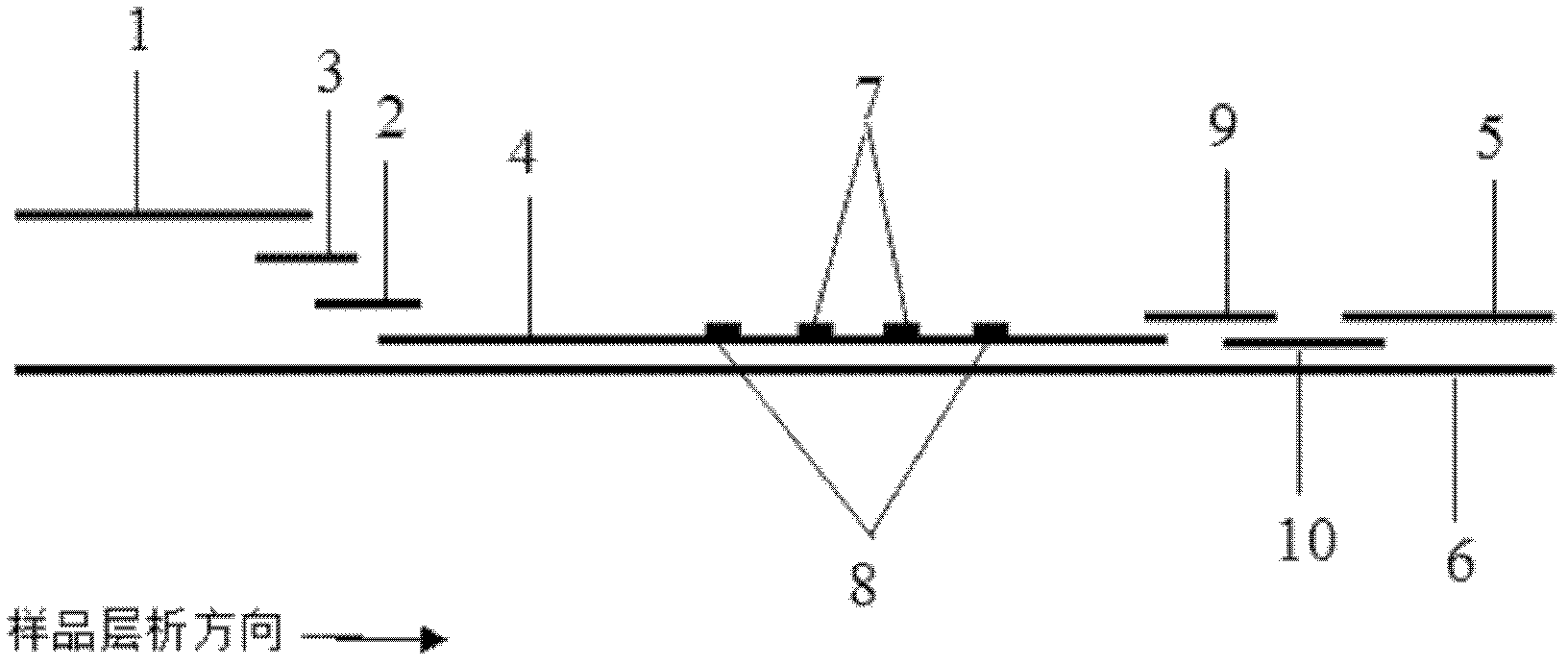
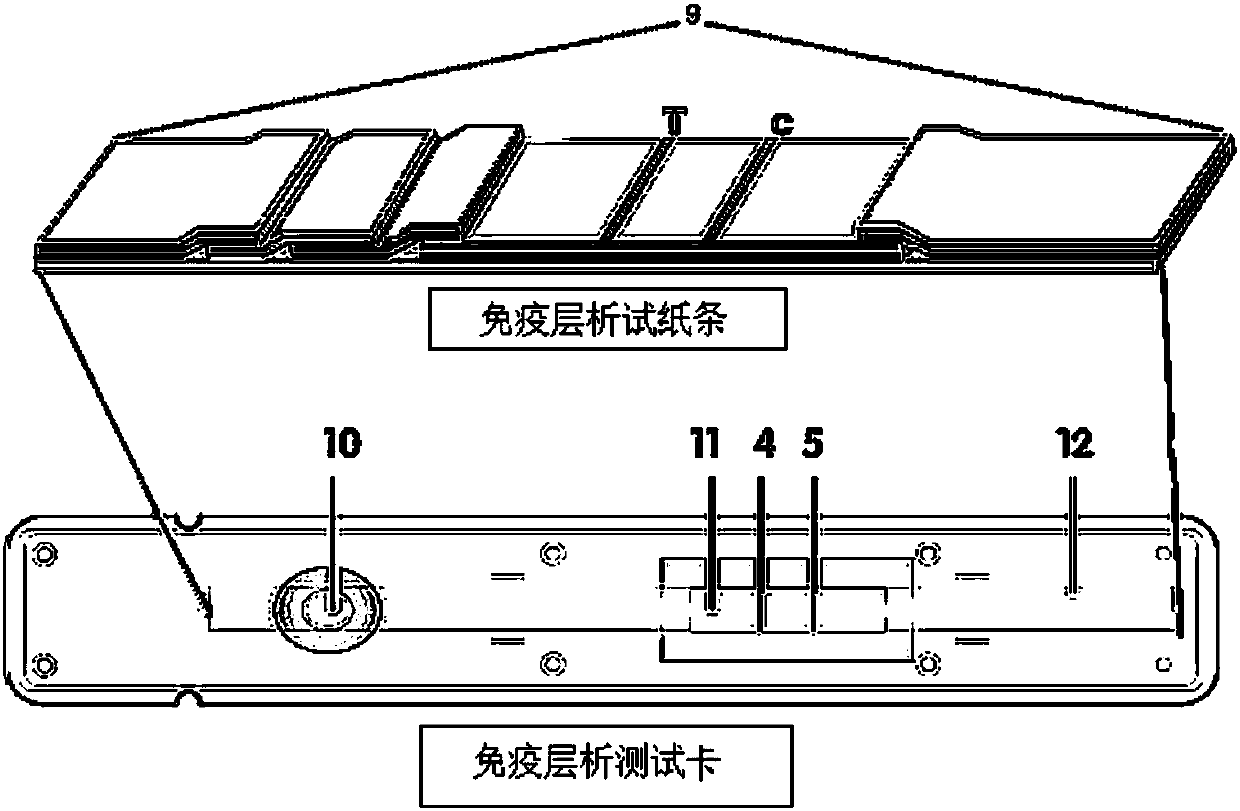
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165ASensitive quantitative detection fastRealize detectionMaterial analysisCritical illnessLinear range
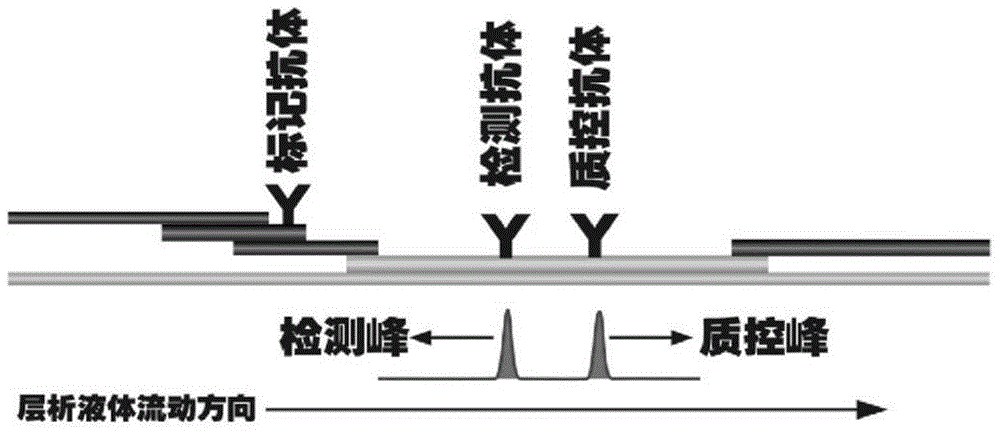
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Fluorescence immunochromatographic assay and kit for quantitative detection of troponin I/creatine kinase isoenzyme/myohemoglobin
ActiveCN102520192AHigh sensitivityHigh detection sensitivityBiological testingNon specificImmunochromatographic Assays
The invention discloses a quantum dot multicolor marking method for quantitative detection of various cardiovascular disease markers and a kit of troponin I / creatine kinase isoenzyme / myohemoglobin. The method realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a multicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the method has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range, small cross interference, and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the troponin I, the creatine kinase isoenzyme and the myohemoglobin simultaneously, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and reagent kit thereof
ActiveCN102539785ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceBasic levelQuantum dot
The invention discloses a fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and a reagent kit thereof. The fluorescent immunochromatography method for the whole quantitative detection of the C-reactive protein (CRP) utilizes excellent fluorescent characteristics of quantum dots, and combines double-color marking technology and immunochromatography technology to achieve fluorescent quantitative detection on the basis of optimizing each constituent elements of test paper. Compared with a conventional colloidal gold immunochromatography method, the fluorescent immunochromatography method for the whole quantitative detection of the CRP has the advantages of being good in stability, low in non-specificity, high in flexibility, wide in linear range and accurate in quantifying. The reagent kit of the fluorescent immunochromatography method can perform the whole quantifying and can simultaneously predict and evaluate infectious diseases, antibiotic effects and cardiovascular and cerebrovascular diseases. The fluorescent immunochromatography method for the whole quantitative detection of the CRP and the reagent kit of the fluorescent immunochromatography method are suitable for various-level hospitals, and particularly contribute to wide popularization in basic-level hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Magnetic fluorescent microsphere immunochromatography quantitative detection method
The invention discloses a magnetic fluorescent microsphere immunochromatography quantitative detection method. In the method, respective excellent characteristics of magnetic nano particles and quantum dots are fully utilized, and an immunochromatography technology is combined to realize fluorescent quantitative detection on the basis of optimizing the structure and ingredients of a test strip. The method has a function of amplifying signals; and compared with the conventional colloidal gold immunochromatography method, the method has the advantages of high mark stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The invention provides a simple, accurate, specific and cheap detection tool for blood samples, urine samples, spittle, excrement and the like, so the method can be widely applied to the fields of medical technology, food safety, veterinary drug residues, environmental monitoring, drug detection and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Fluorescence immunochromatographic assay method for quantitatively detecting heart fatty acid binding protein and kit for quantitatively detecting same
ActiveCN102520194ASolve the backgroundSolve the signal indistinguishableBiological testingBlood plasmaBiology
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay and kit for quantitatively detecting N-terminal pro brain natriuretic peptide
ActiveCN102565423ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceDiseaseN-terminal pro-Brain Natriuretic Peptide
Owner:SHENZHEN KANGMEI BIOTECH
Immunofiltration assay fluorescent quantitative detection method based on high-sensitivity quantum dot
ActiveCN102539771AHigh luminous intensityWide excitation spectrumFluorescence/phosphorescenceQuantum dotQuality control
The invention discloses an immunofiltration assay fluorescent quantitative detection method based on a high-sensitivity quantum dot; the immunofiltration assay fluorescent quantitative detection method comprises the following steps of: constructing a fluorescence immunofiltration array device by using the excellent fluorescent characteristic of the quantum dot in combination of a quantum dot fluorescence labeling technology and an immunofiltration array technology on the basis of optimizing constituent parts for the immunofiltration assay; and after immunofiltration array, detecting the strenght of fluorescent signals of the quantum dot and a quality control dot by using a fluorescence quantometer, correcting the fluorescence strenght of the quantum pot by using the quality control dot, and further realizing the quantitative detection of a tested object according to a standard curve obtained by using the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and high in sensitiveness. Comapred with the conventional collodial gold immunofiltration array method, the immunofiltration assay fluorescent quantitative detection method has the advantages of good labeling stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The method is suitable for samples such as serums, urine, spittle, excrement and the like and can be applicable to the detection of serious illness, poisons, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Rapid nucleic acid amplification system
PendingCN107739710AGood for temperature conversionHigh speedBioreactor/fermenter combinationsHeating or cooling apparatusTemperature controlControl cell
The invention provides a rapid nucleic acid amplification system. Transfer of a nucleic acid amplification reaction solution in a reaction consumable among different temperature zones is performed bya rotating mode and rapid amplification of nucleic acid is realized; the technical problem that an existing nucleic acid amplification system is long in consumed time or the reaction consumable is difficult to realize is solved. The system provided by the invention comprises a disk-shaped or sector reaction consumable, a temperature control module and a rotary supporting assembly. A PCR (Polymerase Chain Reaction) reaction area close to the edge is arranged on the reaction consumable; the temperature control module comprises two or more groups of circumferentially-distributed PCR temperature zone control units and is used for controlling the temperature of the PCR reaction area on the reaction consumable. The reaction consumable is arranged on a supporting component of the rotary supporting assembly; the PCR reaction area is controlled by a rotary motor to transfer between different temperature zone control units and the rapid amplification of the nucleic acid is realized.
Owner:XIAN TIANLONG SCI & TECH
Infertility joint detection kit and detection method thereof
InactiveCN104977400AExcellent detection timeMeet clinical useDisease diagnosisBiological testingRoom temperatureDiluent
The invention discloses an infertility joint detection kit which comprises four reagents including a sample diluent, an enzyme combination, flushing fluid and a substrate. The invention further discloses a detection method for the joint detection kit. The detection method comprises the following steps: balancing the detection kit to room temperature, treating a sample, rinsing the inner film surface of a card window, adding the sample and the flushing fluid, adding the enzyme combination, performing development, ending the operation and the like. The infertility joint detection kit can be used for simultaneously detecting multiple persons in multiple channels, and the sensitivity can reach the detection level of an ELISA product and is much higher than a colloid product; meanwhile, the detection time of the detection kit is close to that of colloid and much shorter than that of ELISA, and operation of a professional is not needed; the infertility joint detection kit is convenient and efficient and can well meet clinical use.
Owner:厦门拜尔杰生物科技有限公司
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
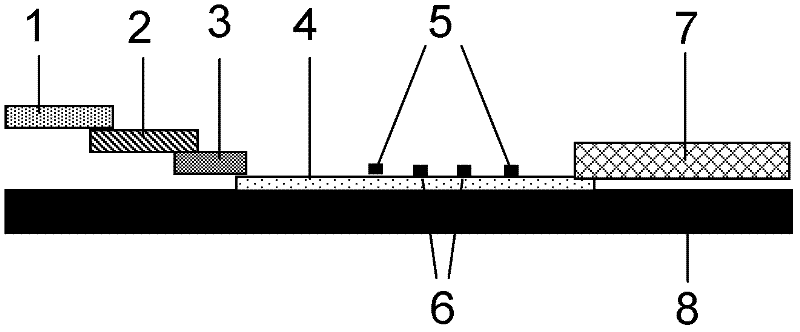
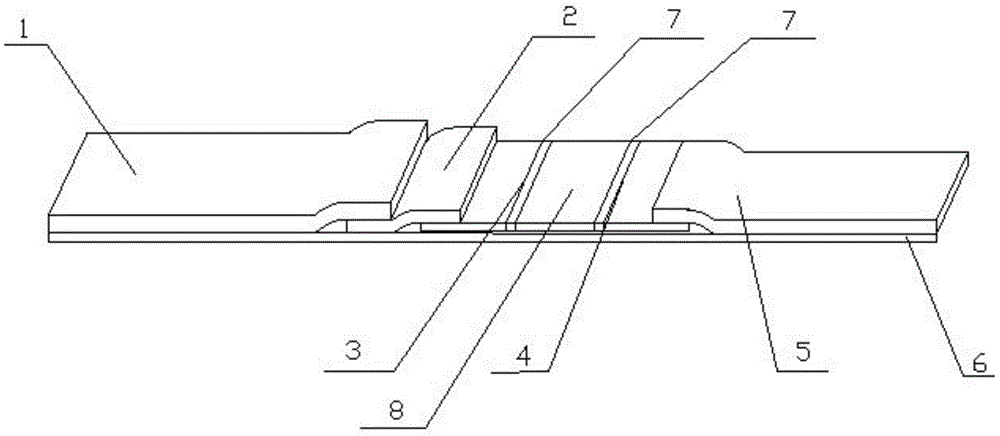
Preparation and application of immunochromatography method and test paper for quantitative detection of prealbumin
InactiveCN106680488AHigh sensitivityImprove capture efficiencyMaterial analysisMicrosphereFluorescence
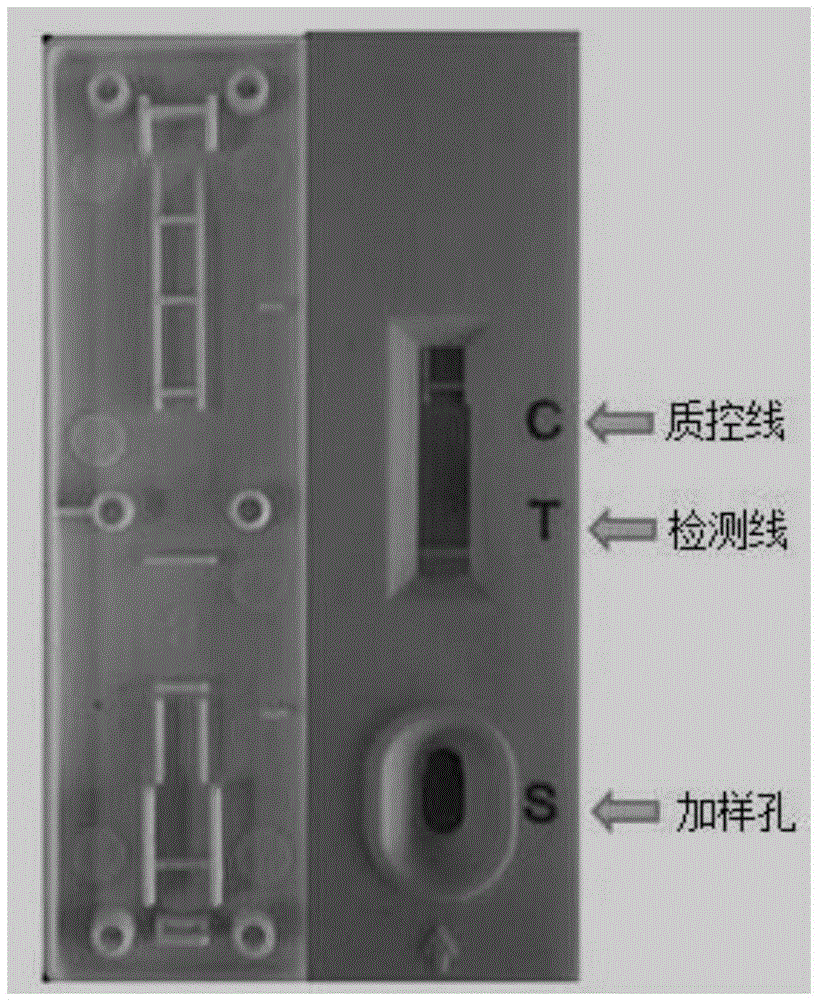
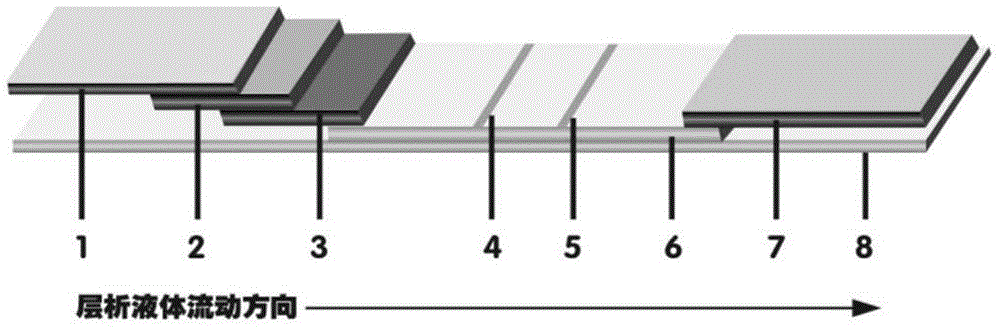
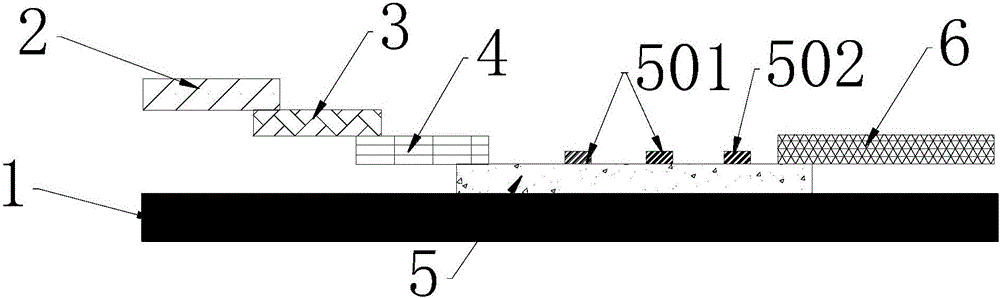
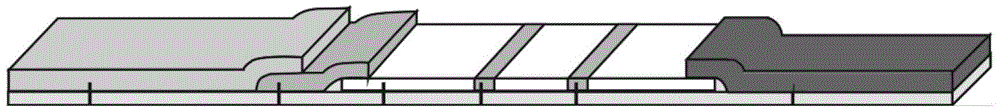
The invention provides preparation and application of an immunochromatography method and test paper for quantitative detection of prealbumin. The test paper consists of a bottom plate, a sample pad, a conjugate pad, a nitrocellulose membrane and a piece of water absorbing paper, wherein the sample pad, the conjugate pad, the nitrocellulose membrane and the water absorbing paper are sequentially adhered to the bottom plate in a lap joint manner and are respectively and partially overlapped; a detection line and a quality control line are arranged on the nitrocellulose membrane; the quality control line is wrapped with a donkey-anti-rat antibody. A method comprises the following steps: (1) adding a solution which is of known concentration and comprises a prealbumin standard substance onto the sample pad of the test paper, and drawing a standard curve; (2) adding a solution of a substance to be tested, performing reaction, and reading content according to standard curves. The test paper and the detection method provided by the invention are high in sensitivity, that is, up to pg / mL, wide in detection linearity, convenient to operate, and short in time, that is, results can be obtained within only 10-20 minutes.
Owner:北京中生金域诊断技术股份有限公司
Fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 and preparation method for fluorescence immunochromatography kit
InactiveCN104655858AHigh luminous intensityWide excitation spectrumDisease diagnosisBiological testingPlasma samplesFluorescence
The invention discloses a fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 by taking fluorescent dye as a marker. The fluorescence immunochromatography kit disclosed by the invention realizes fluorescence quantitative detection for the human epididymis secretory protein-4, has the advantages of being good in stability, wide in linear range, good in specificity, accurate to quantify, simple and quick, can be used for simultaneously detecting whole blood, blood serum and plasma samples, and is suitable for hospitals of various levels.
Owner:DEMAIJI BIOTECH BEIJING
Immune chromatography test paper detection method based on catalyzing and amplifying of detection signal
InactiveCN103063844AHigh detection sensitivityHigh signal resolutionBiological testingNano catalystChemical plating
The invention discloses an immune chromatography test paper detection method based on catalyzing and amplifying of a detection signal. The immune chromatography test paper detection method comprises the following steps: capturing a molecule to be detected to a detecting site through the chromatographic technique; and catalyzing and amplifying the detection signal under the catalyzing effect. According to the immune chromatography test paper detection method based on catalyzing and amplifying of the detection signal, the biomolecule such as immunoglobulin is marked by a catalytic nanometer material; the detection signal is further amplified through an immune chromatography and a chemical plating technology, so that the sensitivity is improved; a large number of catalytic systems of nano-catalysts are provided, the nano-catalysts can catalyze silver, and also can catalyze the settlement of magnetic materials such as cobalt nickel, so that a magnetic signal can be read while the signal is detected, and as a result, the purposes of quantitative detection and semi-quantitative analysis can be realized.
Owner:SOUTHEAST UNIV
Fluorescence immunochromatographic assay and kit for quantitative detection of human cardiac troponin I (cTnI)
ActiveCN102520193ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of cardiac troponin I (cTnI). The fluorescence immunochromatographic assay for quantitative detection of the cTnI realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the cTnI, can be used for detecting whole blood, blood serum and plasma samples simultaneously, and is applied to different levels of hospitals and is particularly favored to be widely popularized to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
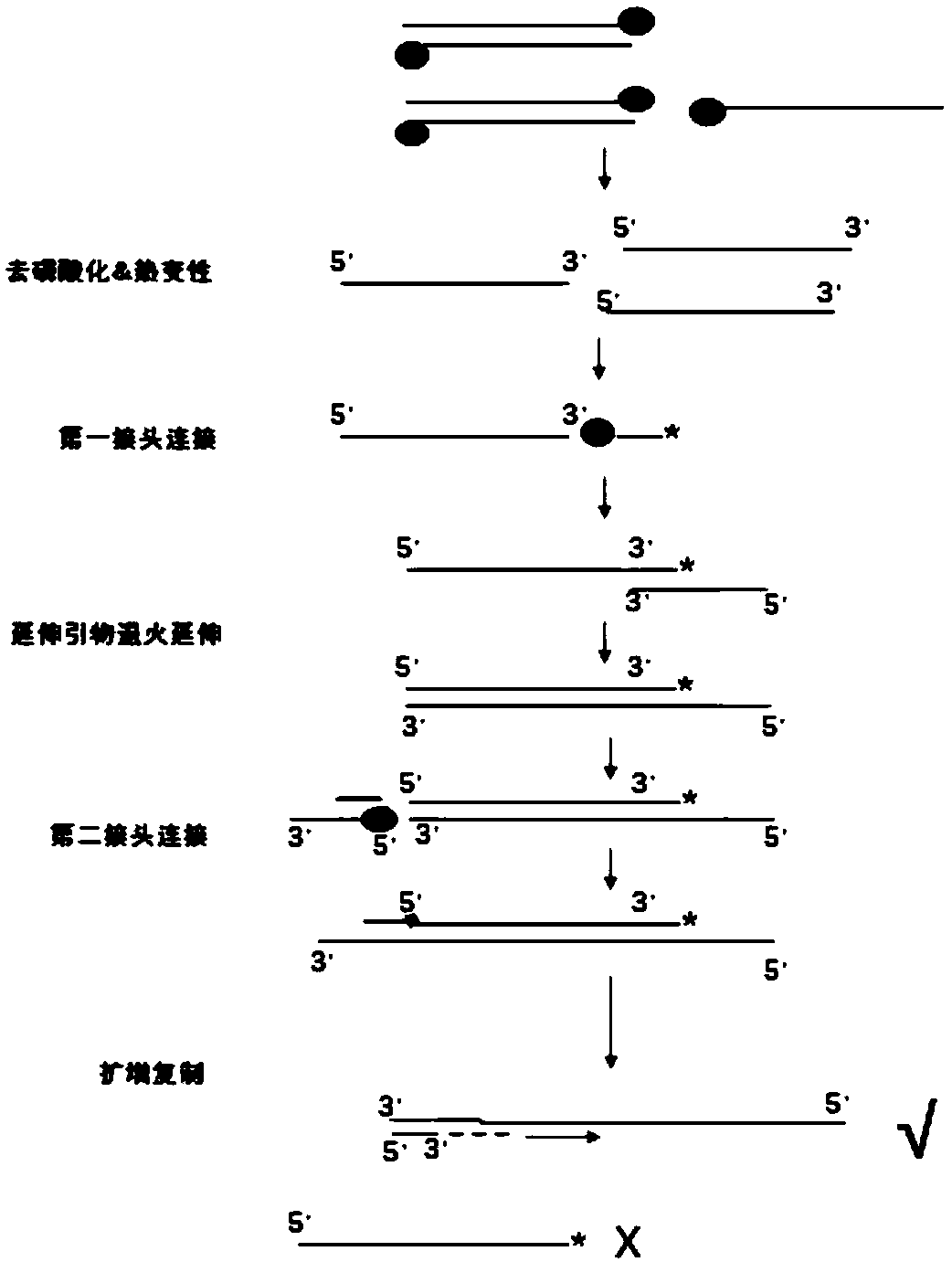
Joint composition and application thereof
ActiveCN110734967AAvoid self connectionAvoid featuresNucleotide librariesMicrobiological testing/measurementNucleotidePhosphorylation
The invention provides a joint composition and application thereof. The joint composition comprises a first joint and a second joint, wherein the first joint is a single-chain joint and contains a UID(unique identifier) sequence and a segment of single-chain nucleotide; the 3'-end of the first joint has sealed modification; the second joint is a double-chain joint formed by a long chain of nucleic acid and a short chain of nucleic acid in complementary pairing; the 5'-end of the long chain of the nucleic acid of the second joint has phosphoric acid modification; and the 5'-end of the short chain of the nucleic acid of the second joint does not undergo phosphorylation modification. Since a synthesized joint with the UID sequence is directly introduced, and the UID sequence is introduced into a double-chain library through extension, a tedious joint preparation process in a conventional UID joint database construction technique is avoided, the experiment efficiency can be improved, theexperiment difficulties can be reduced, and the experiment cost can be reduced.
Owner:MGI TECH CO LTD
NGS database creating primer pool used for amplifying multiple targets in cfDNA sample and application
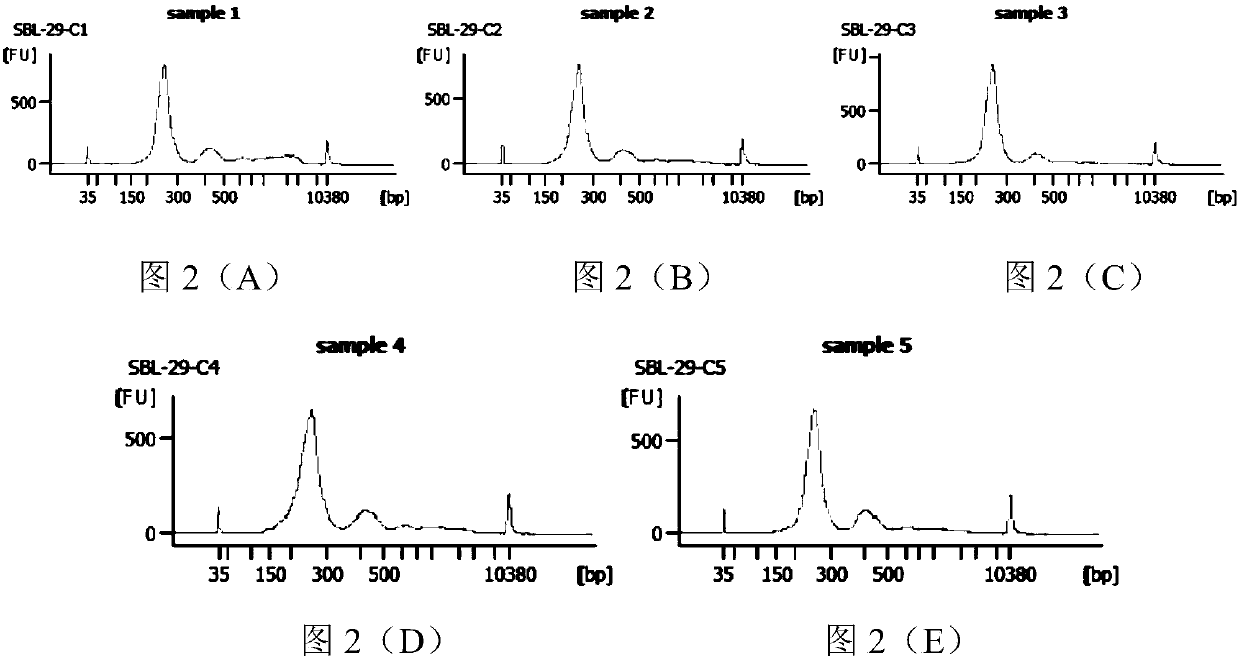
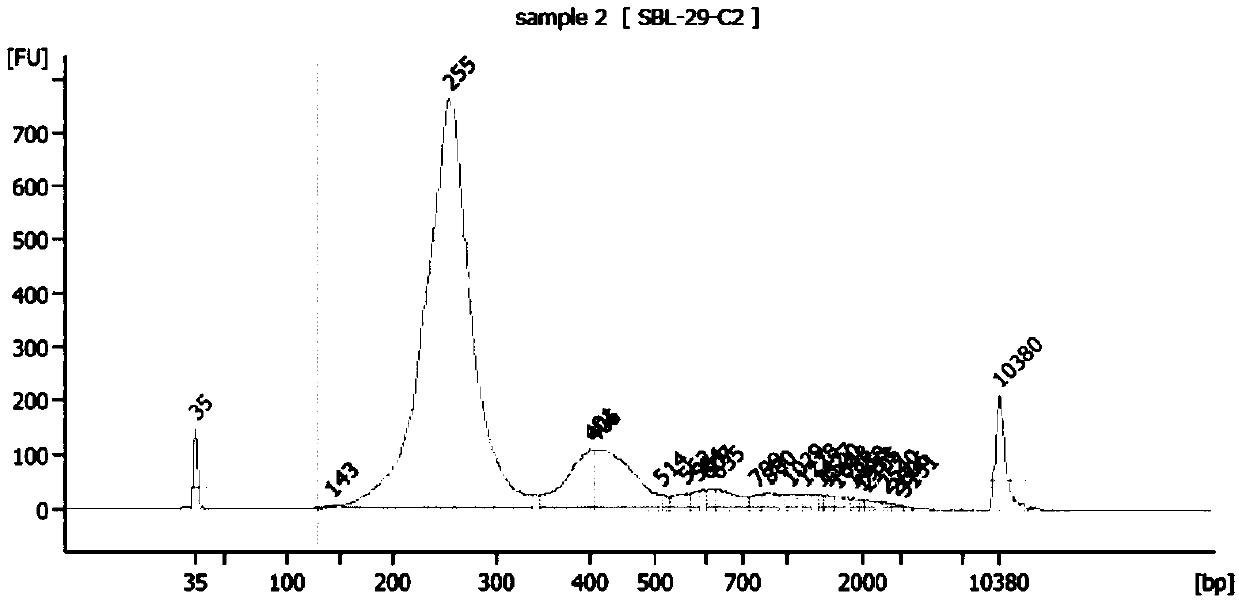
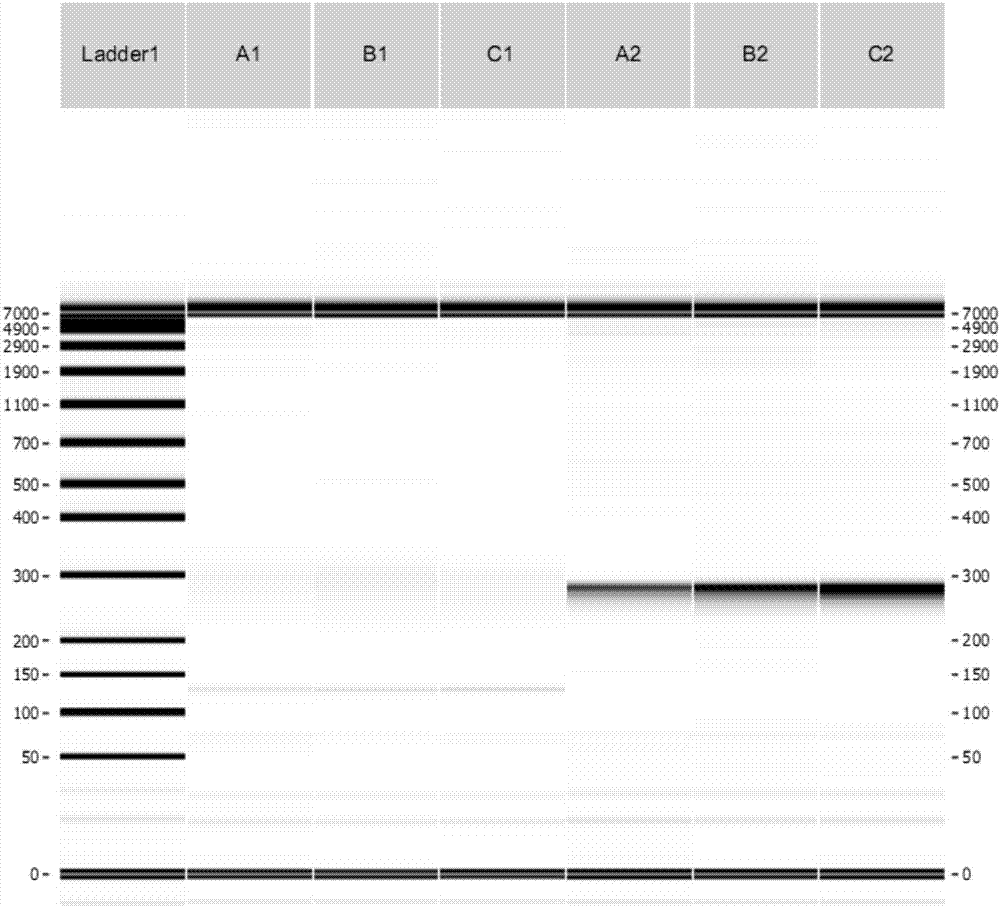
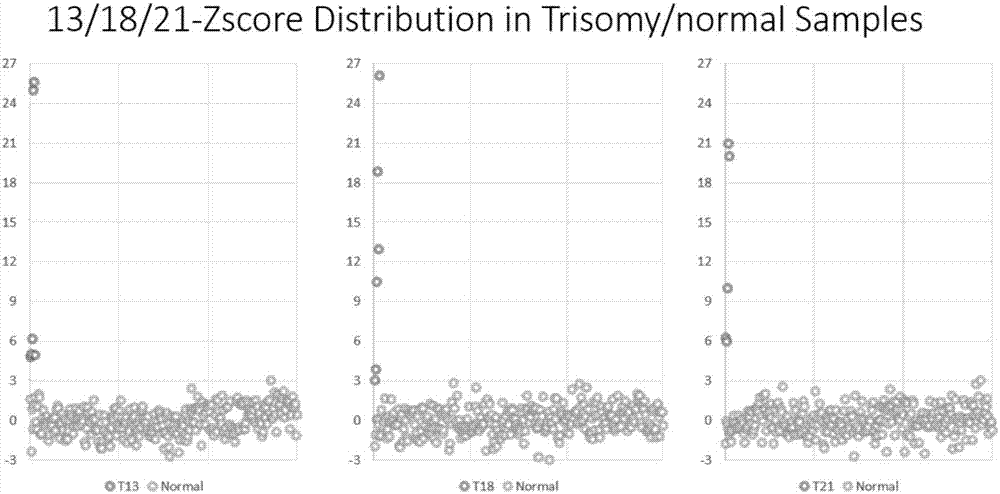
ActiveCN107254541AReduce non-specificityImprove scalabilityMicrobiological testing/measurementDNA/RNA fragmentationMultiplex pcrsEcology
The invention discloses an NGS database creating primer pool used for amplifying multiple targets in a cfDNA sample and application. Each primer in the primer pool is composed of a universal primer segment and a specific primer segment, wherein the universal primer segment is connected with a terminal 5' of a specific primer, a (N)k sequence used for adjusting a primer Tm is connected between the universal primer segment and the specific primer segment of at least one primer, and difference between Tm values of the primers is not more than 10 DEG C. The primers in the primer pool have almost the same Tm, non-specific amplification is lower, further amplification is easy, and the number of covering layers in a detection target area can be reached conveniently. The method has the advantages that thousands of PCR can be carried out in the same system at the same time, and the limit that the conventional multiple PCR generally does not exceed 100 is broken.
Owner:广州万德基因医学科技有限公司
Kit for rapidly and quantitatively detecting troponin and creatine kinase isozyme
ActiveCN106198997ASimple and fast operationRapid responseDisease diagnosisBiological testingBiotin-streptavidin complexCreatine kinase
The invention belongs to the field of kits, and particularly relates to a kit for rapidly and quantitatively detecting troponin and creatine kinase isozyme, and a preparation method of the kit. The kit comprises a base plate, wherein a sample pad, an antibody binding pad I, an antibody binding pad II, an enveloped analysis film and a water absorption pad which are sequentially lapped are arranged on the base plate; an immunomagnetic bead which is coupled with biontin-marked anti-troponin I monoclonal antibody is enveloped on the antibody binding pad I, an immunomagnetic bead which is coupled with an anti-creatine kinase isozyme monoclonal antibody is enveloped on the antibody binding pad II, and the surface of the magnetic bead I is enveloped with streptavidin. The kit has the advantages of being simple and convenient to operate, fast to react, high in sensitivity, strong in specificity, suitable for on-site detection and the like, and is suitable for large-range popularization and application.
Owner:ANHUI IPROCOM BIOTECH CO LTD
Process for refining gulonic acid
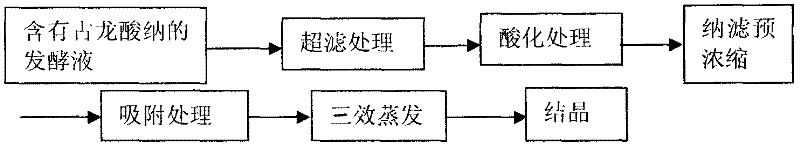
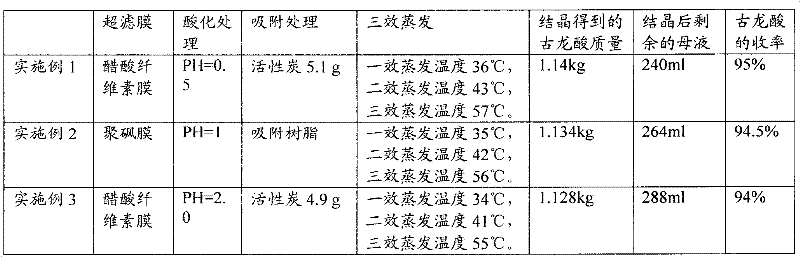
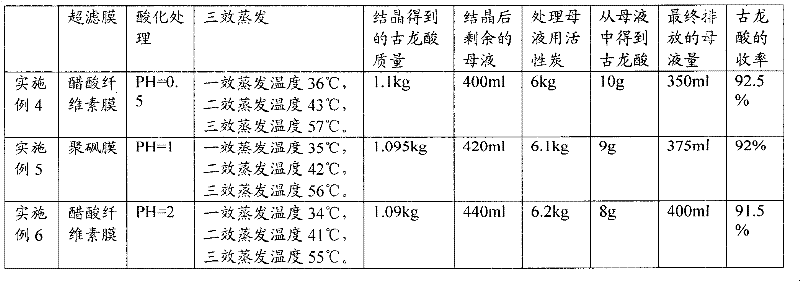
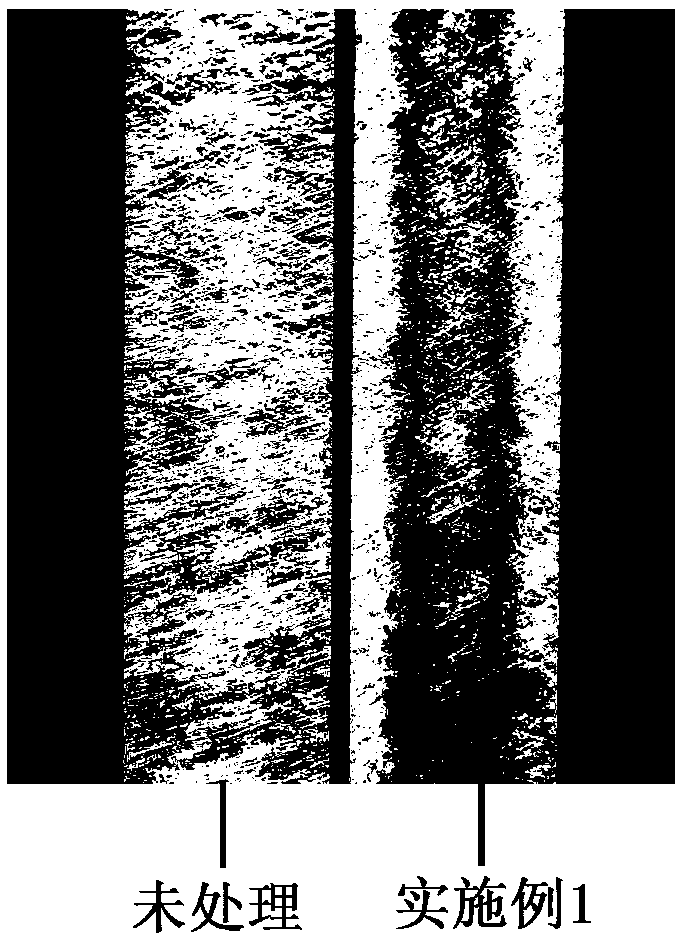
InactiveCN102391101AImprove hydrophobicityReduce non-specificityPreparation from carboxylic acid saltsCarboxylic compound separation/purificationUltrafiltrationIon exchange
The invention discloses a process for refining gulonic acid. The process comprises the following steps of: performing ultrafiltration on fermentation liquor containing sodium gulonate; acidizing the fermentation liquor subjected to the ultrafiltration by using an ion exchange device, wherein the pH value of the fermentation liquor in the ion exchange device is below 2.0; performing nanofiltration preconcentration on the acidized fermentation liquor to obtain preconcentrated liquor; adsorbing the preconcentrated liquor by using active carbon or adsorbent resin; and performing triple effect evaporation on the adsorbed preconcentrated liquor to obtain concentrated liquor, and crystallizing the concentrated liquor to obtain a gulonic acid crystal. In a refining process route, the fermentation liquor is subjected to the ultrafiltration, ion exchange, the nanofiltration preconcentration to remove a large number of macromolecular impurities and soluble protein, and the preconcentrated liquor subjected to the nanofiltration preconcentration is adsorbed to remove impurities such as residual sugar, the soluble protein, pigments and the like further, so the quality of feed liquor before crystallization is optimized, the purity and yield of products are improved, and the discharge capacity of mother liquor is reduced.
Owner:HEBEI THINK DO ENVIRONMENT CO LTD
Preparation and application of Golgi protein 73 (GP73) antigen silicon-based magnetic bead conjugate
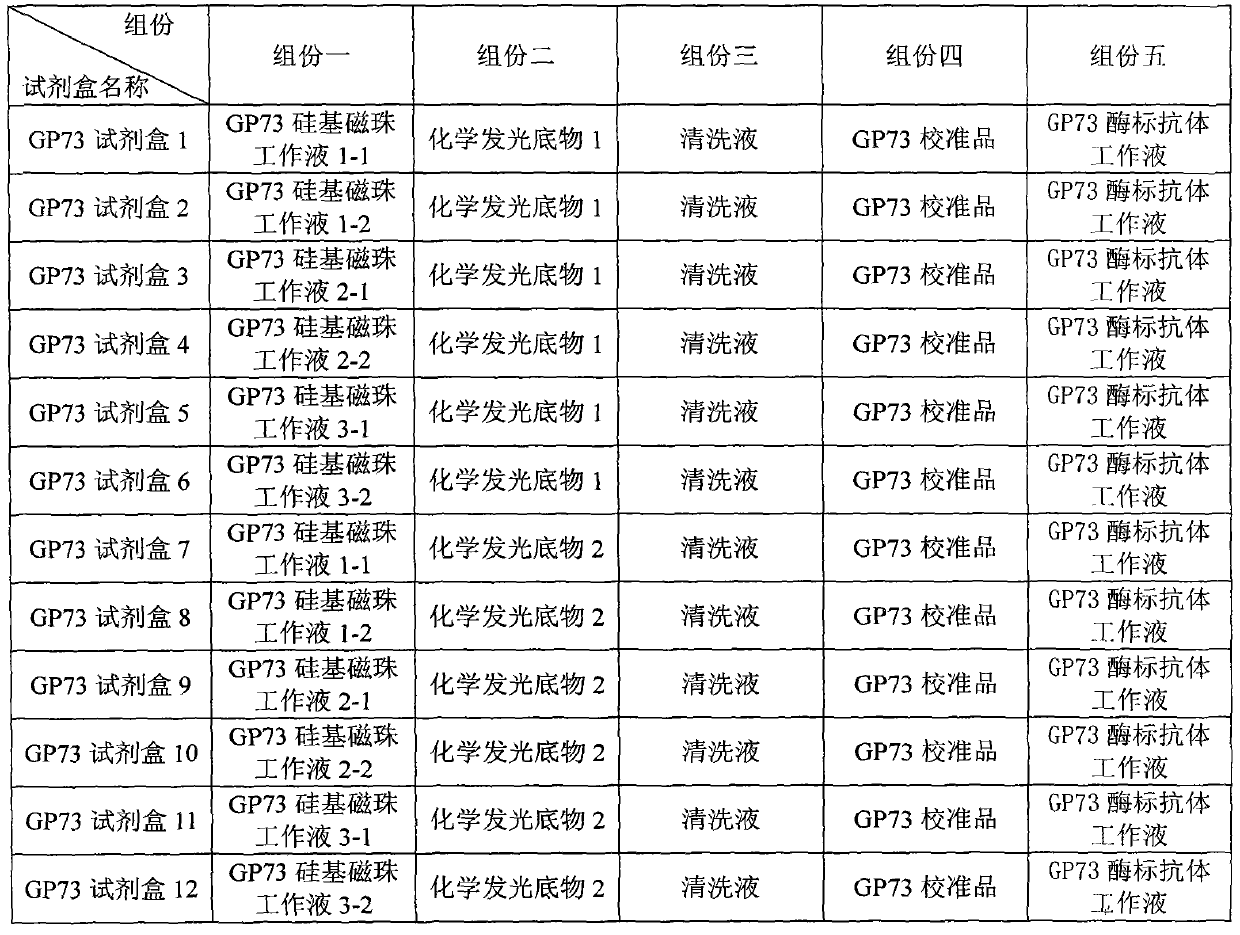
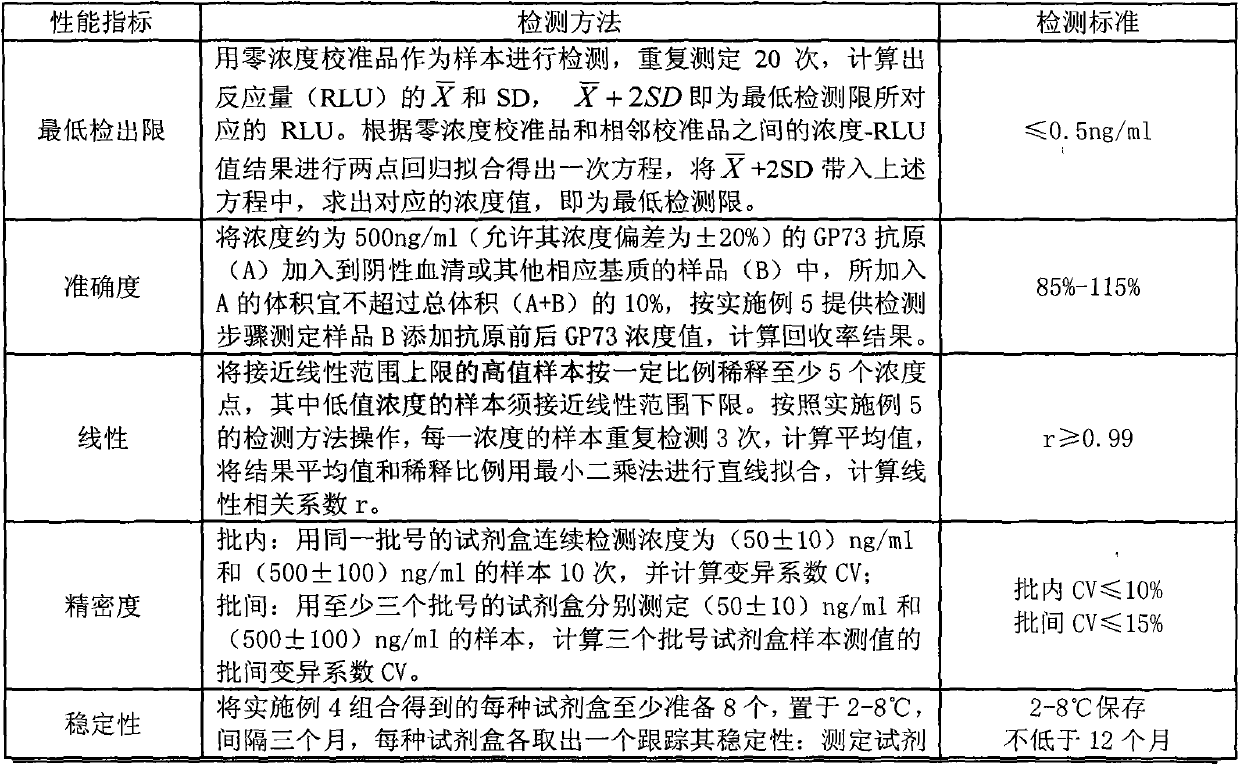
ActiveCN104198721AGood stabilityReduce non-specificityBiological testingAntigenPrimary hepatic carcinoma
The invention provides a GP73 antigen silicon-based magnetic bead conjugate as well as a competition method GP73 quantitative determination kit formed by taking the conjugate as one of the components and matching with other components. The kit disclosed by the invention consists of a Golgi protein 73 (GP73) antigen silicon-based magnetic bead conjugate working solution, a Golgi protein 73 (GP73) calibration material, an alkaline phosphatase labeled Golgi protein 73 (GP73) monoclonal antibody working solution, a cleaning solution and a chemiluminescence substrate. Meanwhile, the invention provides a method for preparing the GP73 antigen silicon-based magnetic bead conjugate and a method for preparing each component in the kit. The method comprises the following steps: preparing the GP73 antigen silicon-based magnetic bead conjugate working solution, preparing an enzyme-labeled antibody, preparing the calibration material, preparing the cleaning solution and preparing the chemiluminescence substrate. The kit provided by the invention can be mainly applied to diagnosis of primary hepatic carcinoma and has the advantages of high sensitivity, wide linear range, hook effect avoidance, low cost, short detection time consumption and the like.
Owner:北京惠中医疗器械有限公司 +2
Protein chip for lyme disease flagellin antigen immunoserology diagnosis and preparation method and application of protein chip
InactiveCN104374921AHigh sensitivityStrong specificityBiological testingAgainst vector-borne diseasesSerodiagnosesADAMTS Proteins
The invention discloses a protein chip for lyme disease flagellin antigen immunoserology diagnosis and a preparation method and application of the protein chip. The protein chip is characterized in that borrelia burgdorferi recombination flagellin antigen probes are fixed on the surface of a solid phase carrier in a dot matrix mode; the solid phase carrier is a 16-amino-1-hexadecyl mercaptan modified gold foil chip which is combined with 4-(N-maleinimide methyl) cyclohexane-1-carboxylic acid succinimide ester and 4-(dimethylamino) pyridine. The protein chip disclosed by the invention can accurately detect an anti-flagellin antigen IgG antibody and an anti-flagellin antigen IgM antibody in serums of lyme disease patients, the operation is simple, and the detection result is stable.
Owner:ANHUI MEDICAL UNIV
Fluorescence immunochromatographic assay and kit for quantitative detection of creatine kinase isoenzyme (CK-MB)
ActiveCN102520173ASolve the backgroundSolve the signal indistinguishableMaterial analysisDiseaseCreatine kinase
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of acute myocardial infarction marker-creatine kinase isoenzyme (CK-MB). The fluorescence immunochromatographic assay for quantitative detection of the CK-MB realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the CK-MB, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Rapid library building method for identifying target protein chromatin binding map
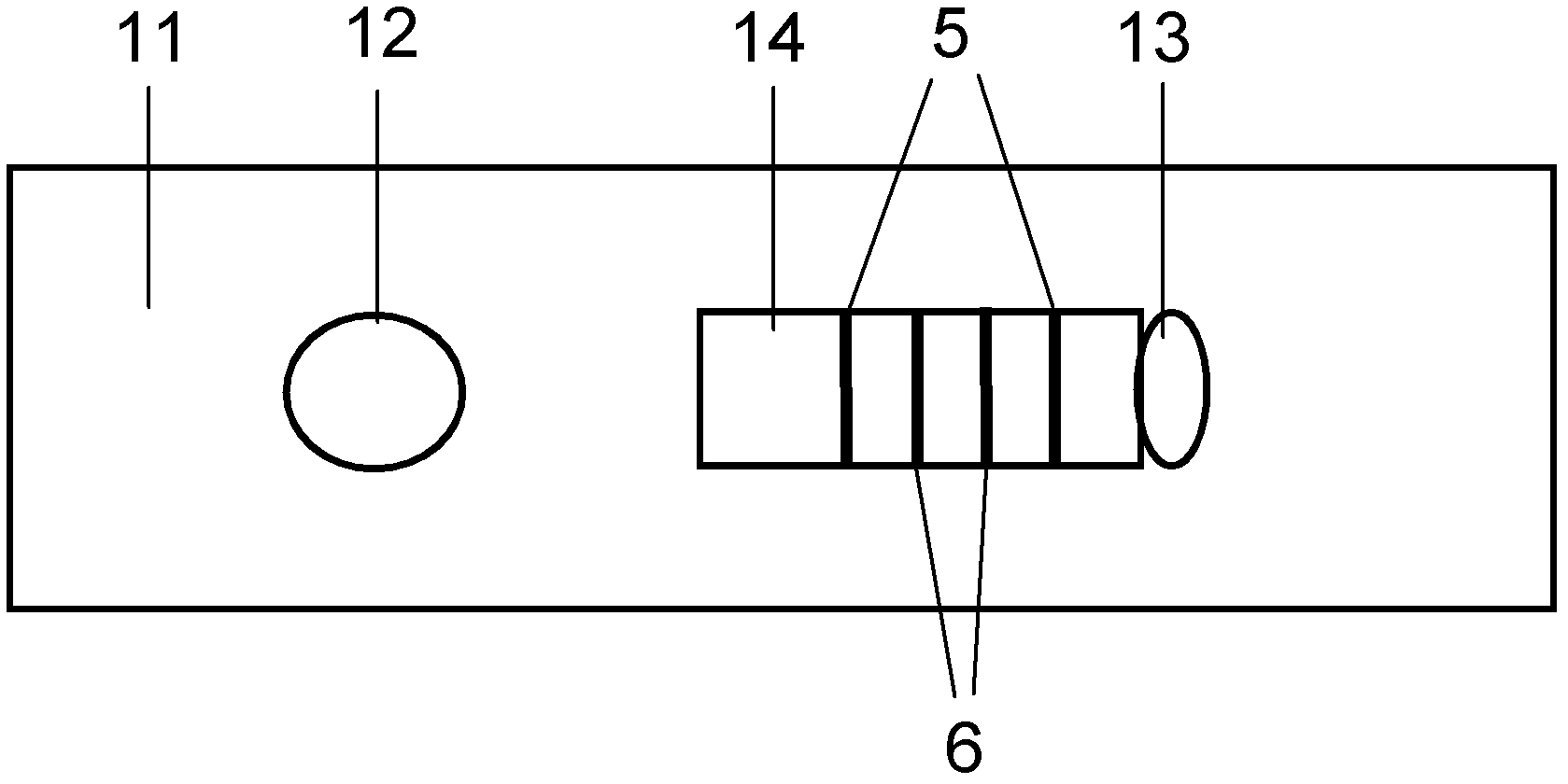
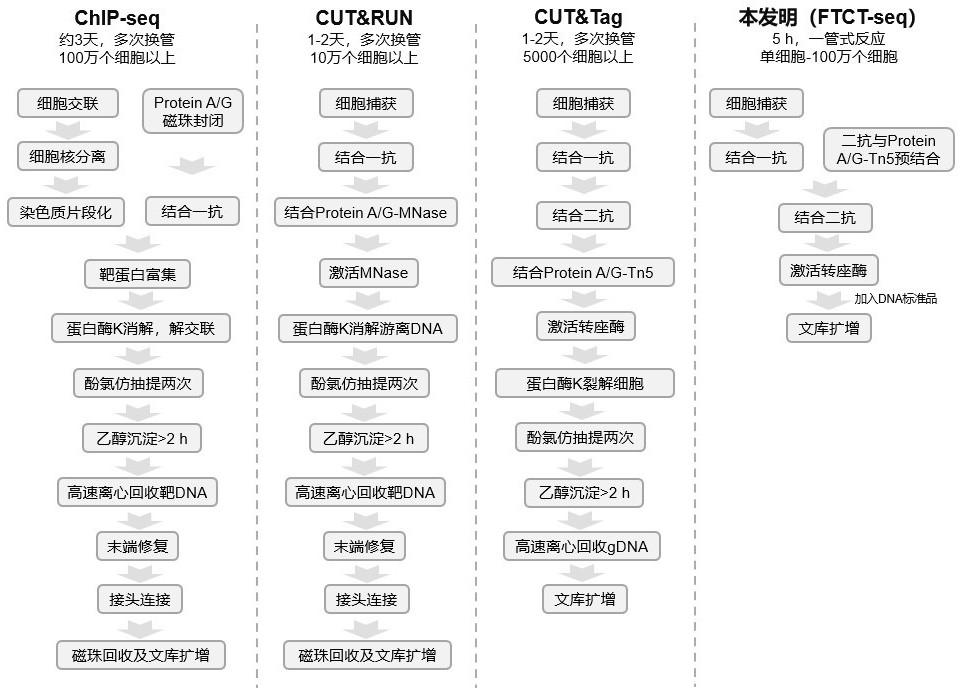
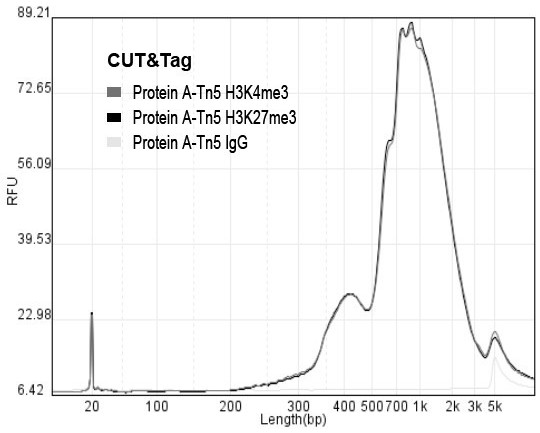
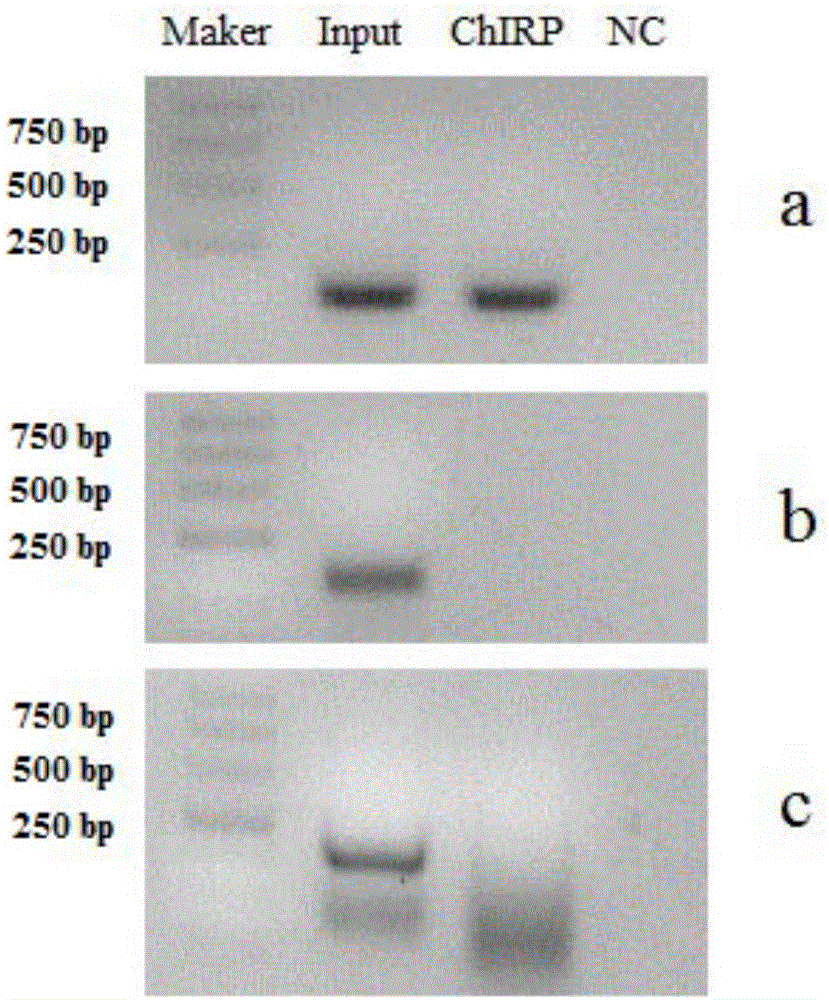
ActiveCN112553695ALower requirementReduce non-specificityLibrary creationProtein targetMagnetic bead
The invention discloses a rapid library building method for identifying a target protein chromatin binding map, which comprises the following steps: collecting sample cells, adding activated magneticbeads into the cells, incubating, and incubating the magnetic beads with a primary antibody; pre-binding a secondary antibody with recombinant fusion transposase; incubating the magnetic beads with the pre-combined secondary antibody and recombinant fusion transposase, adding a transposase activator and a cell perforating agent into the magnetic beads, and incubating; and terminating the transposase reaction, and carrying out library amplification. According to the method, the process of a traditional method is remarkably simplified, the library building time is shortened, the library buildingefficiency is improved, the loss is small, and the requirement for library building materials is lower. Meanwhile, three DNA standard substances with different concentrations are doped into the library building process, so that the effect of accurately quantifying the DNA copy number in the library can be achieved. The method is very suitable for researching a small amount of precious samples orsamples with different treatment states.
Owner:YEASEN BIOTECHNOLOGY (SHANGHAI) CO LTD
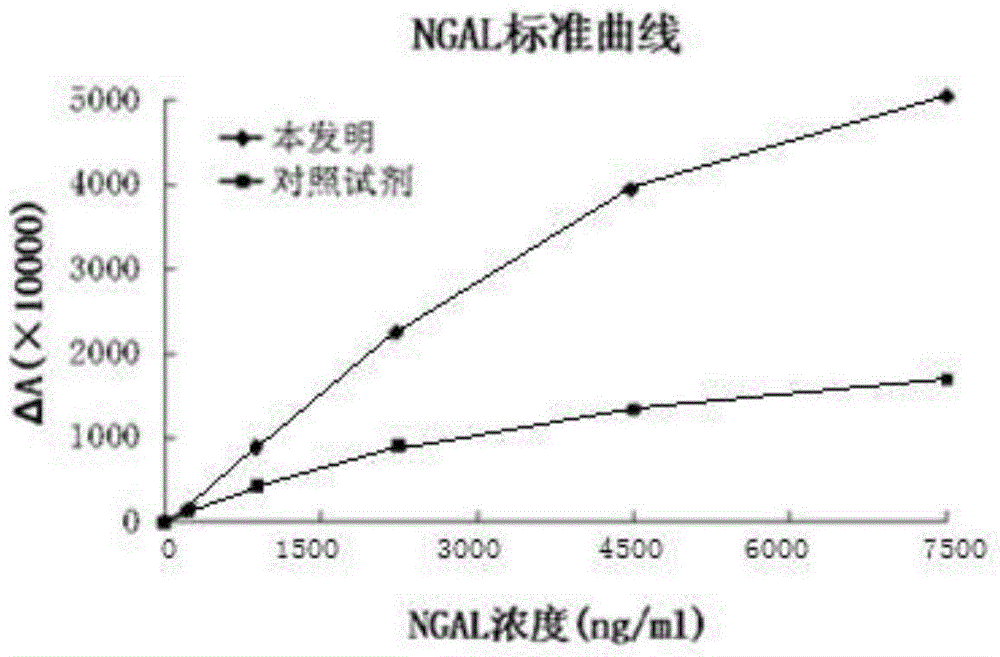
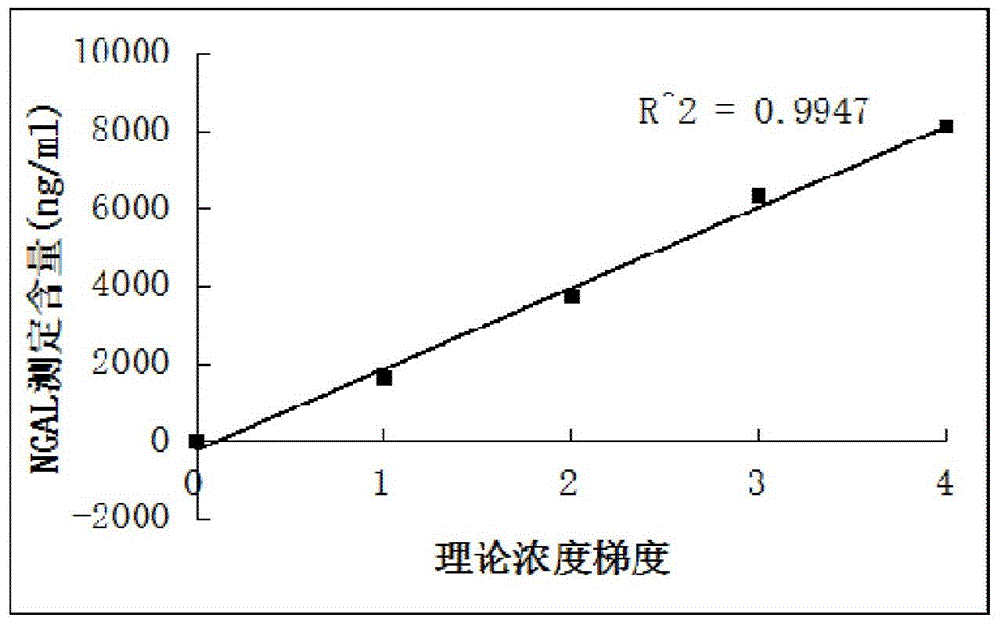
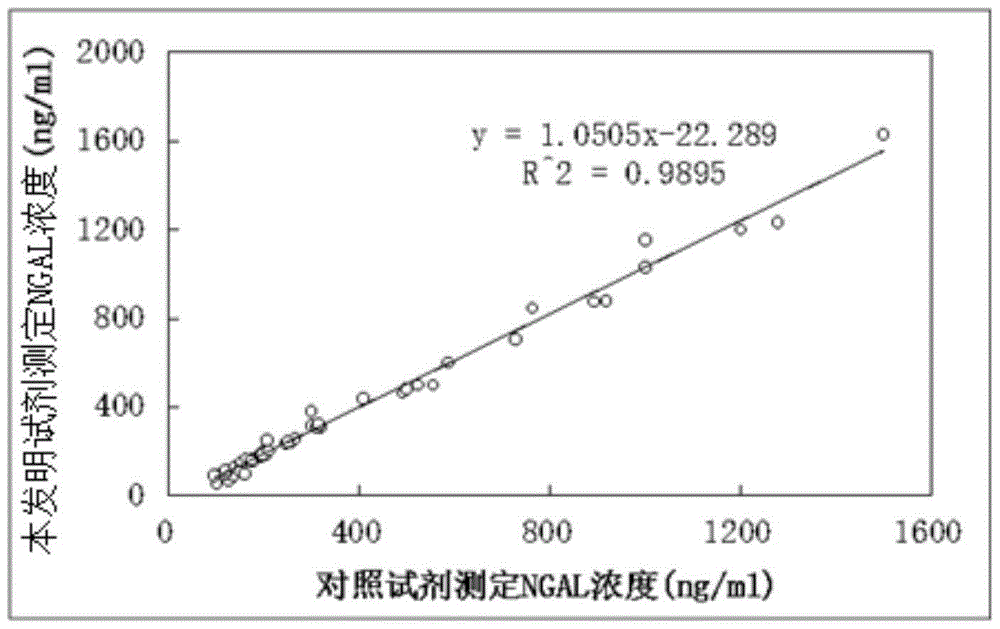
Kit for content detection of neutrophil gelatinase-associated lipocalin
The invention discloses a kit for content detection of neutrophil gelatinase-associated lipocalin, wherein a reagent R1 comprises Tris, NaCl, BSA, Tween-20, PEG and NaN3; a reagent R2 comprises Tris, NaCl, BSA, Tween-20, NaN3, sucrose and NGAL antibody sensitized latex particles; a NGAL reagent reference standard sample comprises Tris, NaCl, BSA, Tween-20, EDTA, NaN3 and NGAL of different contents; the NGAL antibody sensitized latex particles comprise PS nanometer latex particles and PVN nanometer latex particles, and the particle size of the PS nanometer latex particle is larger than the particle size of the PVN nanometer latex particle. The invention has the advantages of simple reagent composition, good test sensitivity, wide linearity range, good stability, low test cost and high precision, and the kit is convenient for popularization.
Owner:NINGBO RUI BIO TECH
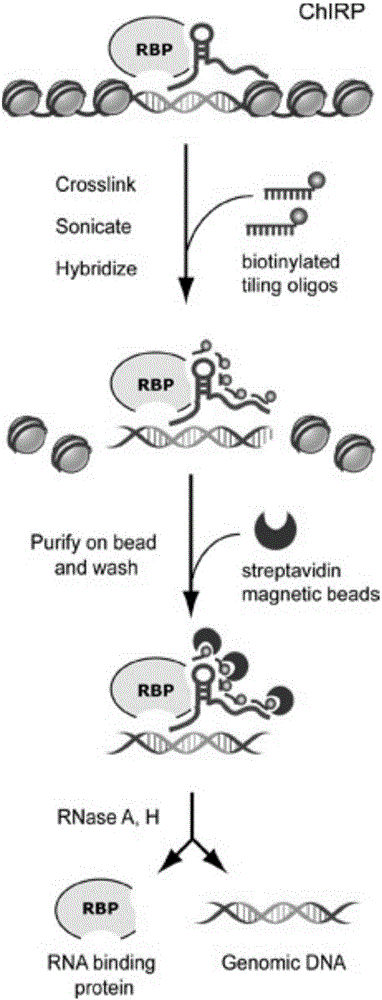
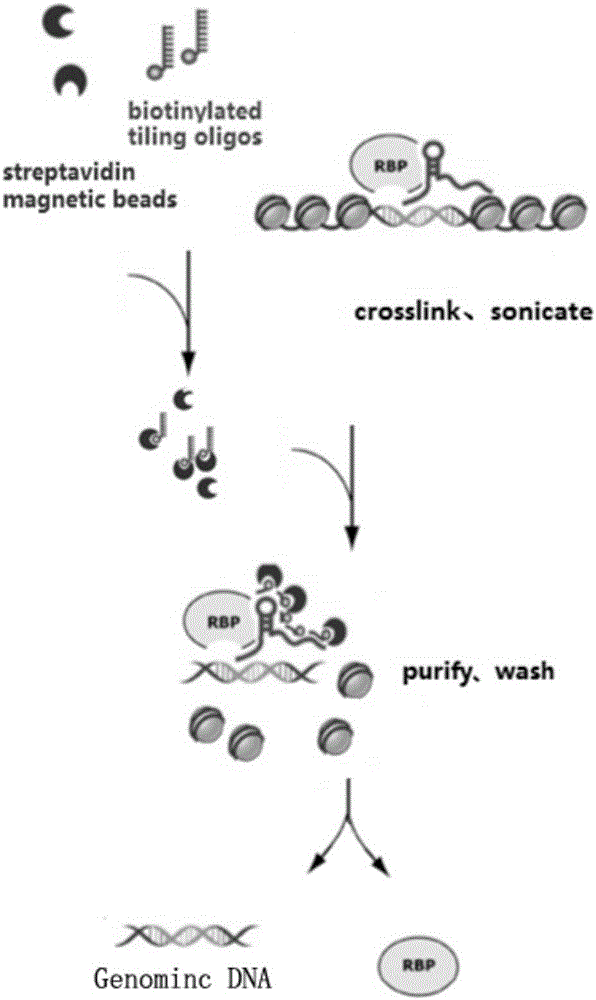
RNA (ribonucleic acid)purification chromatin separation technique
PendingCN106191296AReduce non-specificityReduce non-specific bindingMicrobiological testing/measurementLysisMagnetic bead
The invention relates to an RNA (ribonucleic acid) purification chromatin separation technique. The technique includes the following steps: binding probes with magnetic beads prior to hybridizing with chromatin lysis solution, and then performing washing for multiple times via gradient temperature. The magnetic beads are closed with BSA and random oligonucleotide primers in advance, and accordingly nonspecific binding of the magnetic beads with proteins and RNA is reduced. The magnetic beads are incubated with the probes firstly prior to being incubated with the proteins after excessive probes are removed, gradient temperature washing is performed after hybridization to assure that the magnetic beads fully bind with the probes and bind with target RNA, and hybridization efficiency is increased.
Owner:GUANGZHOU BIOSENSE BIOSCI
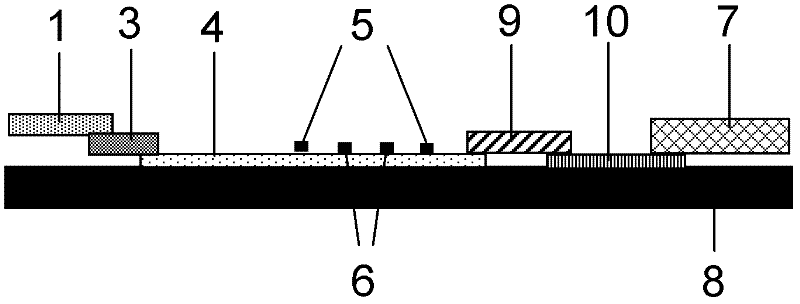
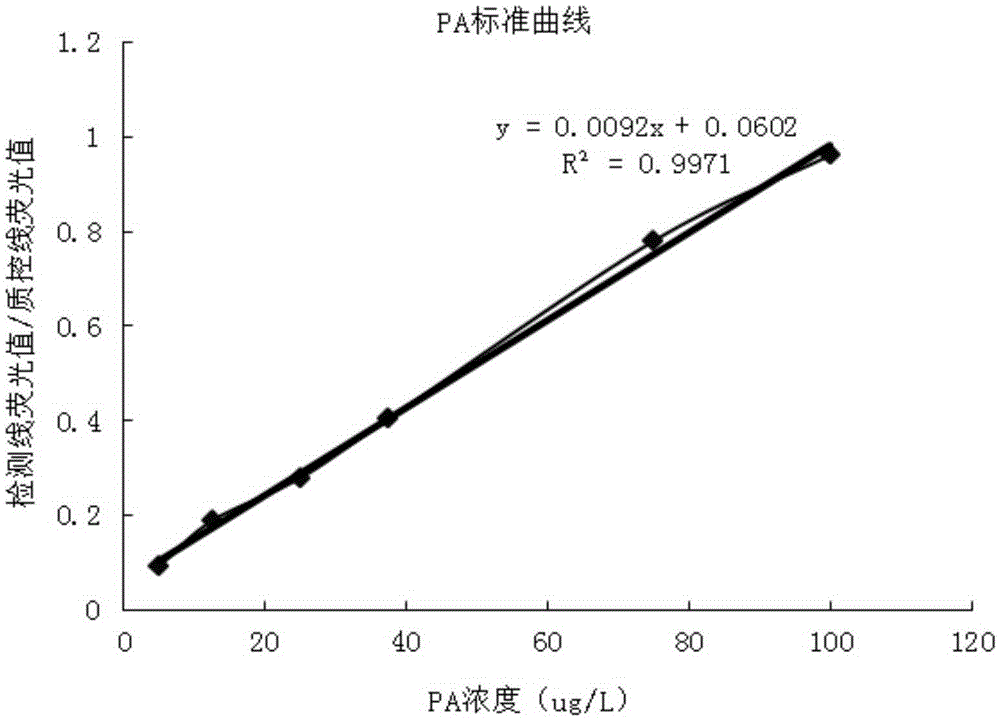
Method and kit for quantitative combined detection of PA (Prealbumin) and CRP (C-reactive Protein), as well as preparation method and application of kit
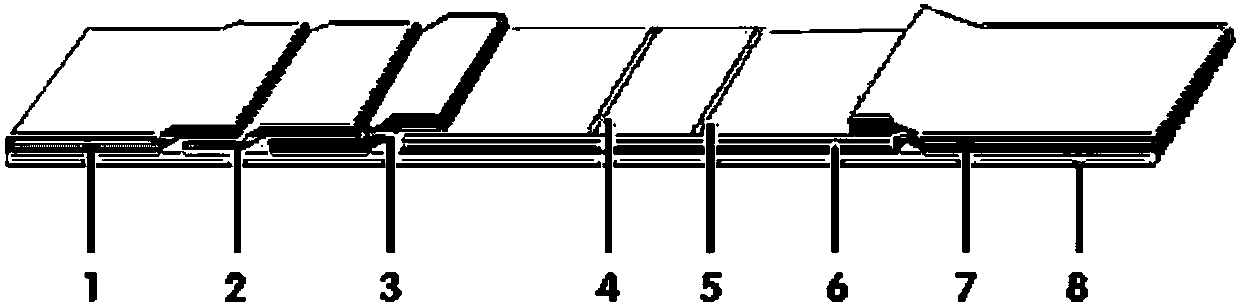
InactiveCN106680508AHigh sensitivityImprove capture efficiencyBiological testingFluorescence/phosphorescenceQuality controlEngineering
The invention provides a method and a kit for quantitative combined detection of PA (Prealbumin) and CRP (C-reactive Protein), as well as a preparation method and application of the kit. The kit comprises a test paper strip I and a test paper strip II, which are used for detecting PA and CRP, wherein the test paper strip I is composed of a bottom plate I, a sample pad I, a combination pad I, a nitrocellulose membrane I and water absorption paper I; and the test paper strip II is composed of a bottom plate II, a sample pad II, a combination pad II, a nitrocellulose membrane II and water absorption paper II. The detection method comprises the following steps: (1) adding a PA standard object into the sample pad I and adding a CRP standard object into the sample pad II; after reacting, drawing a standard curve according to a specific value of the fluorescence intensity of a detection line and the fluorescence intensity of a quality control line; and (2) adding a solution of an object to be detected into the sample pad I and the sample pad II, and reading PA content and CRP content in the solution of the object to be detected according to the standard curve. The kit and the detection method, provided by the invention, are simple and convenient to operate, high in clinical acceptance degree and good in detection effect.
Owner:北京中生金域诊断技术股份有限公司
Two-photon fluorescence immunochromatography kit for quantitative determination of anti-Mullerian hormone (AMH) and preparation method of kit
InactiveCN107621540ALong fluorescence lifetimeHigh luminous intensityMaterial analysisQuantitative accuracyBlood plasma
The invention discloses a two-photon fluorescence immunochromatography kit for quantitative determination of anti-Mullerian hormone (AMH), which utilizes fluorescent dye as a maker. The two-photon fluorescence immunochromatography kit, realizing fluorescence immunochromatography quantitative determination, has the advantages of good stability, wide linear range, good specificity, high sensitivity,high quantitative accuracy and easy and quick operation, can be applied to detection of whole blood samples, serum samples and plasma samples simultaneously, and is applicable to medical treatment ofhospital at different levels and family practice.
Owner:DEMAIJI BIOTECH BEIJING
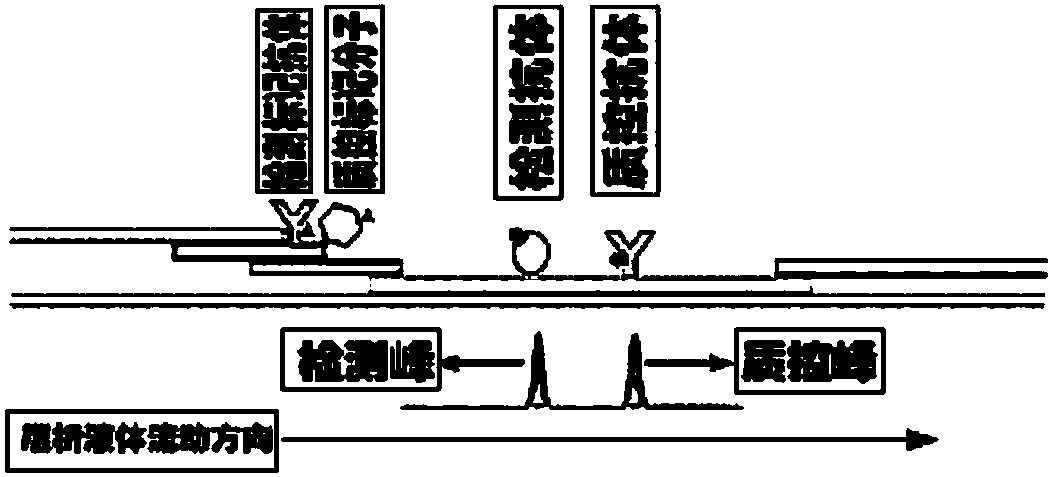
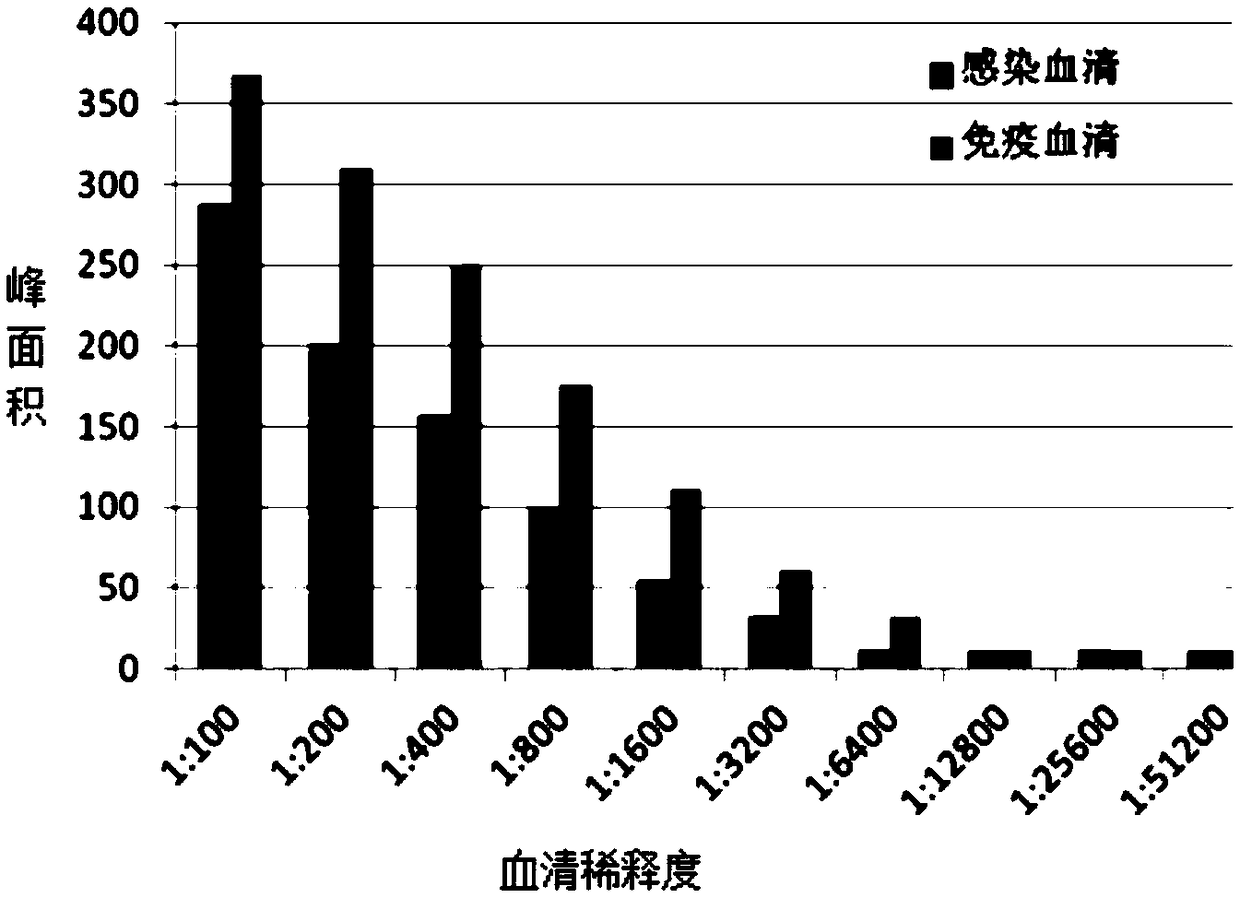
Bigeminal test strip for foot-and-mouth disease virus immune antibody evaluation and infection-immunity differential diagnosis
The invention discloses a bigeminal test strip for foot-and-mouth disease virus immune antibody evaluation and infection-immunity differential diagnosis. The test strip includes a detection line 1 printed with a foot-and-mouth disease virus VP1 epitope polypeptide artificial antigen, and a detection line 2 printed with a foot-and-mouth disease virus non-structural protein epitope polypeptide artificial antigen. The artificial antigens prepared according to the invention have the advantages of easy availability, stable structure, purity up to 99%, low cost, rapid mass production, etc., thus solving the shortcomings of low expression level of traditional expression protein, difficult guarantee of a natural spatial structure, difficult renaturation, difficult removal of mycoprotein, influenceon the detection result specificity and the like in traditional expression protein, and also solving the risk of incomplete virus inactivation in use of inactivated viruses. The invention also performs pretreatment on a gold labelled pad, thus being more conducive to water absorption of the gold labelled pad and release of gold labelled protein, and effectively solving the problems of slow and incomplete release of gold labelled protein, affection to the test paper product detection accuracy, sensitivity, shelf life and the like in existing test paper.
Owner:HENAN ACAD OF AGRI SCI
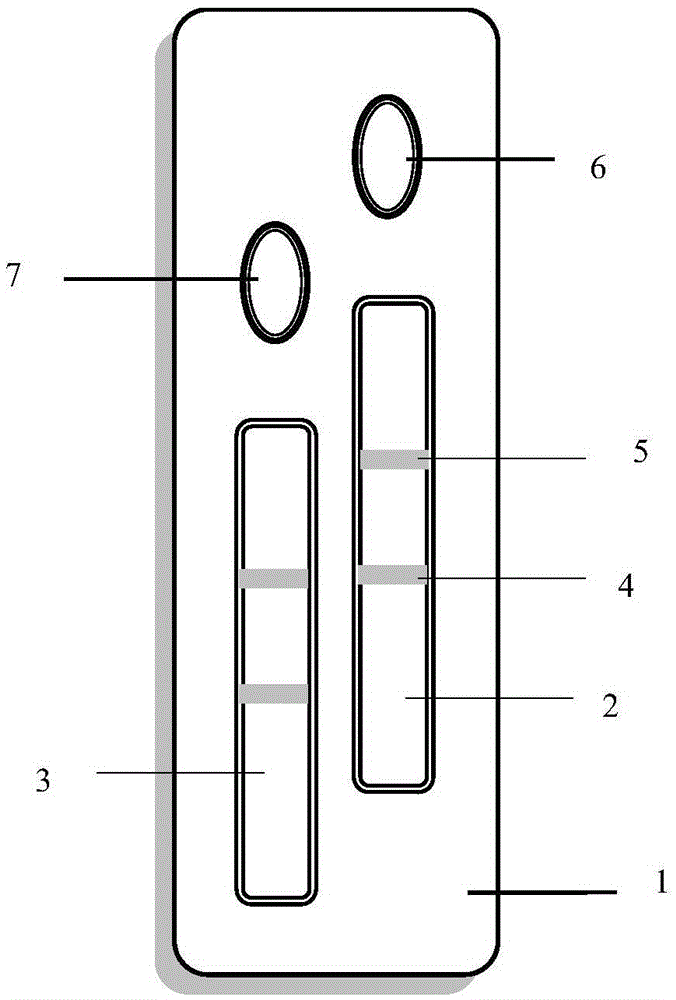
Test strip for rapid cervical cancer HPV detection device
The invention provides a test strip for a rapid cervical cancer HPV detection device. The test strip comprises a bottom plate, a sample pad, a bonding pad and an absorption pad, wherein the sample pad is arranged above on end of the bottom plate; the absorption pad is arranged above the other end of the bottom plate; the bonding pad is overlapped below one end of the sample pad; a nitrocellulose membrane pad is arranged between the bonding pad and the absorption pad; a test line and a control line are arranged on the nitrocellulose membrane pad; and nucleic acid probes are respectively arranged on the test line and the control line. The test strip has the advantages that 1, the sensitivity is high, namely according to the design of the nucleic acid probes, the sensitivity of the detection method can be greatly improved during application; and 2, the specificity is high, compared with high nonspecific binding interference of antigens-antibodies, due to the design of the specific nucleic acid probes, the nonspecific action in a sample can be greatly reduced during actual application.
Owner:HENAN KELONG MEDICAL APP & INSTR
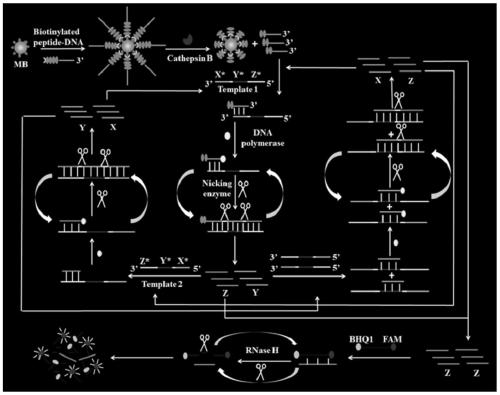
Biosensor for detecting cathepsin B as well as detection method and application thereof
ActiveCN111172235AEnables ultra-sensitive detectionHigh amplification efficiencyMicrobiological testing/measurementBiological material analysisCathepsin BA-DNA
The invention provides a biosensor for detecting cathepsin B as well as a detection method and application thereof, and belongs to the technical field of detection analysis. The biosensor at least comprises a polypeptide-DNA conjugate, a DNA template 1 and a DNA template 2. First primer DNA containing an amino acid residue is converted into new primer DNA containing no amino acid residue, the steric hinerance effect in a nucleic acid amplification process can be completely eliminated, and the amplification reaction efficiency can be improved. Meanwhile, proceeding of multiple chain replacementreactions and the circulating digestion function of ribonuclease H can further amplify a fluorescence signal, so that ultra-sensitive detection of the activity of cathepsin B can be realized, and thebiosensor has good practical application values.
Owner:SHANDONG NORMAL UNIV
Difunctional ion chelated magnetic carrier, and applications thereof
ActiveCN107828776AUnique biomedical activityReduce non-specificityCarrier-bound/immobilised peptidesOn/in organic carrierMicrosphereElution
The invention belongs to the technical field of enzyme purification and immobilization, and more specifically relates to a curdlan-based protein purifying / immobilizing difunctional ion chelated magnetic carrier, and applications thereof. The difunctional ion chelated magnetic carrier is obtained via activation and chelating of magnetic curdlan microspheres, and possesses immobilizing and purifyingfunctions; according to magnetic curdlan microspheres, Fe3O4 nanoparticles are taken as a core, a shell is obtained via curdlan reverse embedding, and the difunctional ion chelated magnetic carrier is obtained via synthesis. The difunctional ion chelated magnetic carrier possesses magnetic responsibility; in applications, column separation is not needed, separation of the difunctional ion chelated magnetic carrier from a reaction system can be realized easily under the action of an applied magnetic field, and one-step elution and recycling can be realized rapidly and conveniently. The difunctional ion chelated magnetic carrier is capable of playing an important role in the fields such as enzyme immobilization and enzyme purification because applications are convenient and high in efficiency; the stability and catalytic efficiency of xylanase obtained via immobilization using the difunctional ion chelated magnetic carrier are increased obviously, production cost is reduced, and large scale industrialized production is promising to realize.
Owner:SHENYANG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com