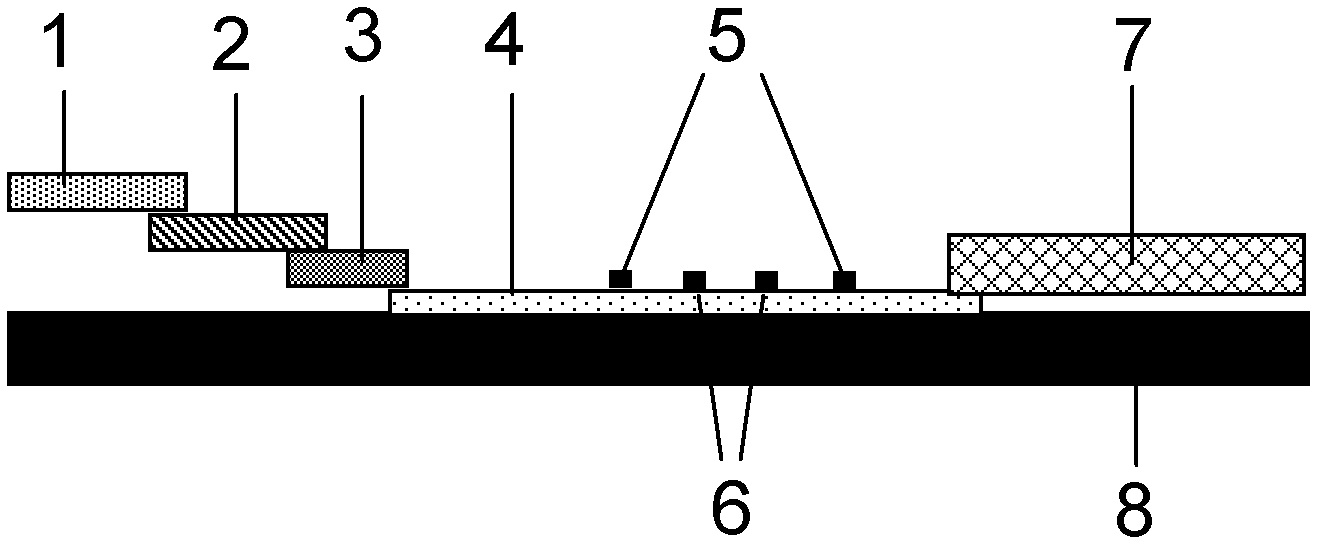
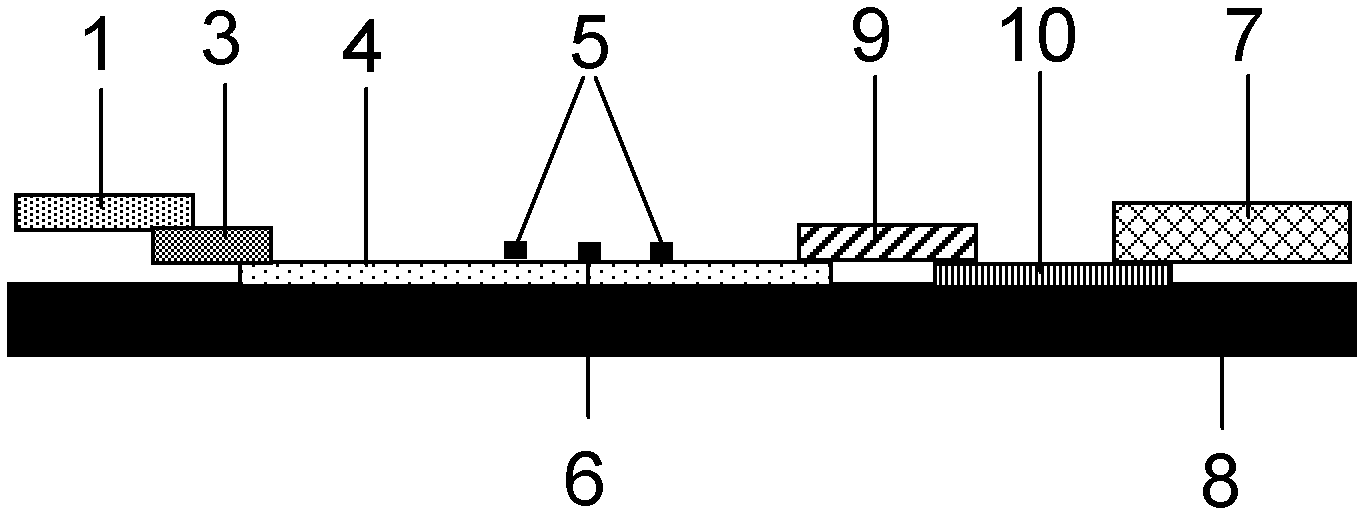
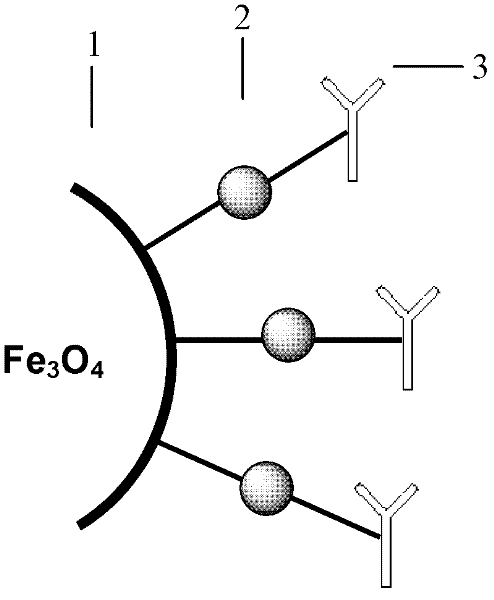
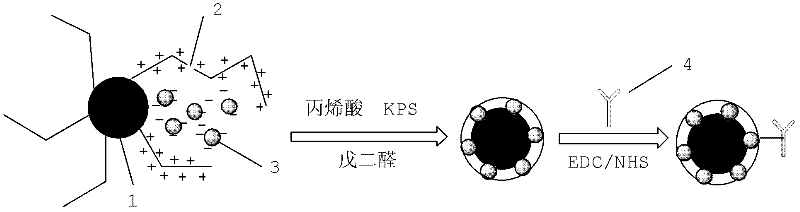
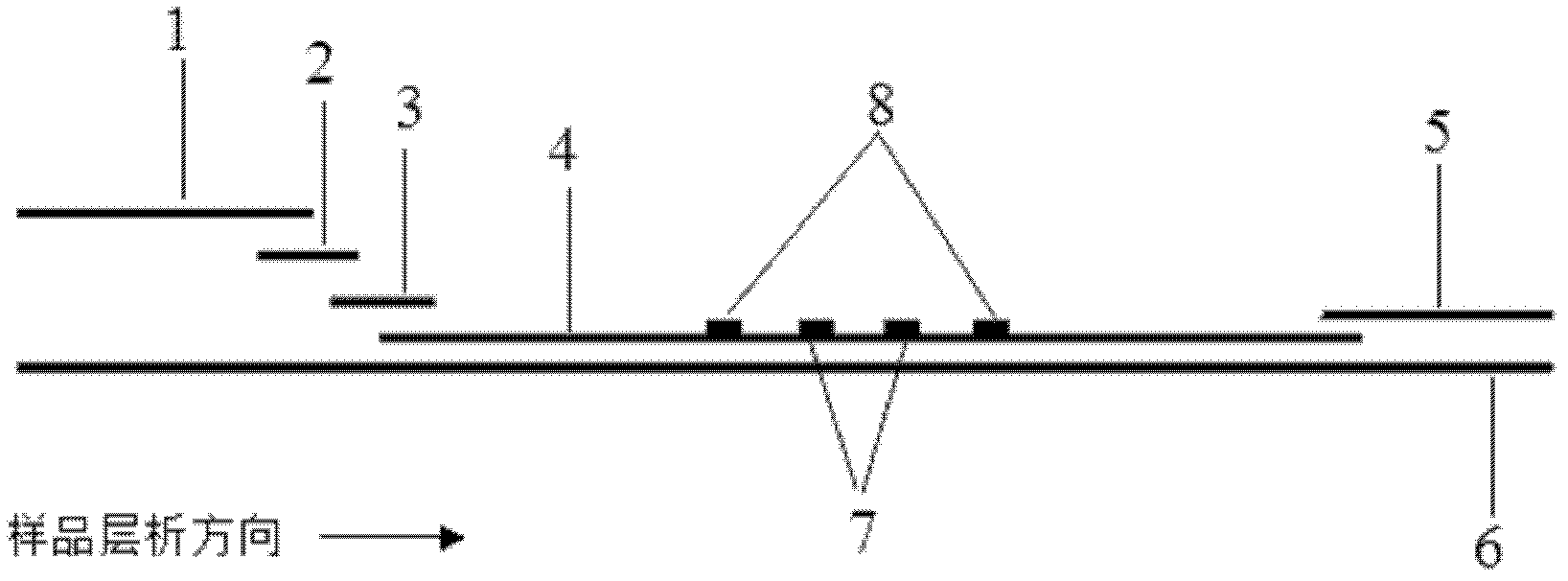
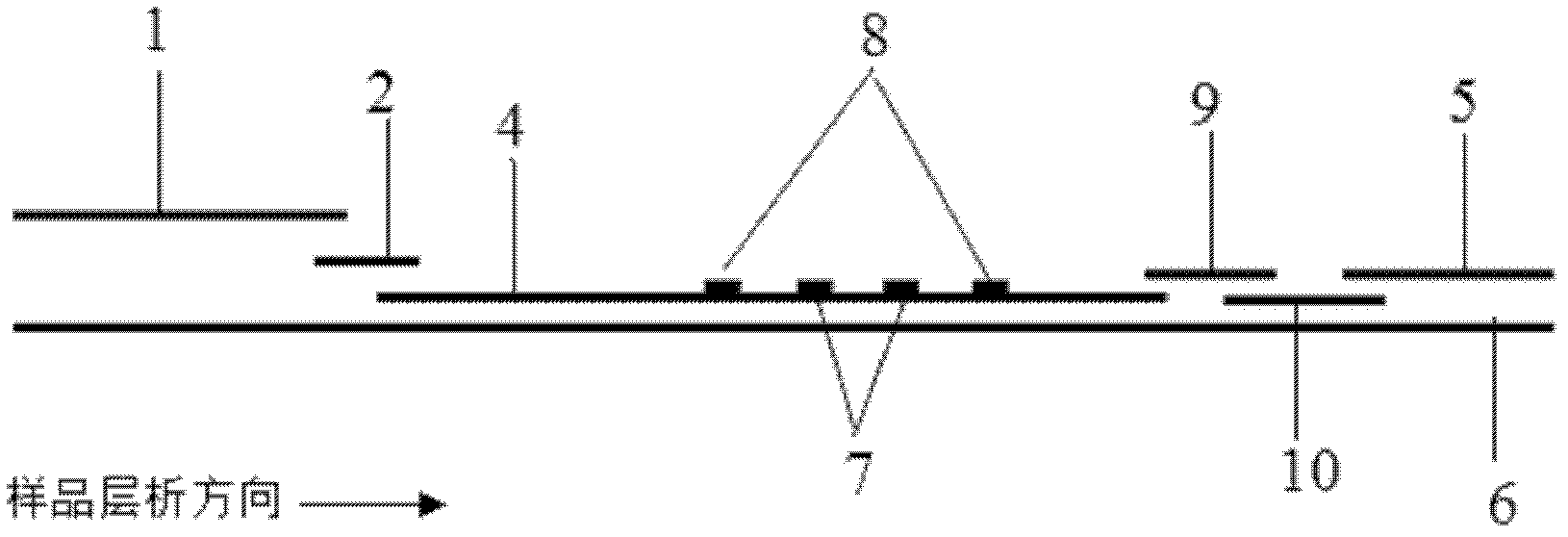
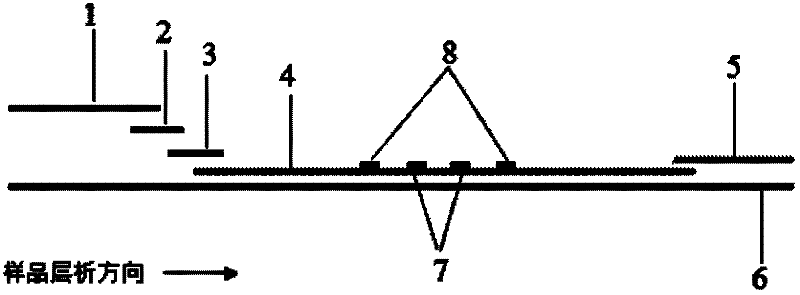
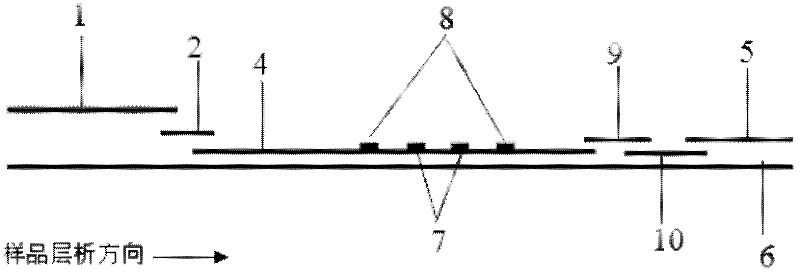
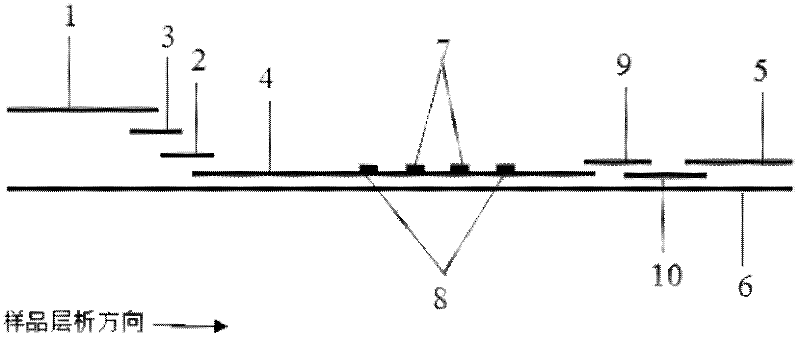
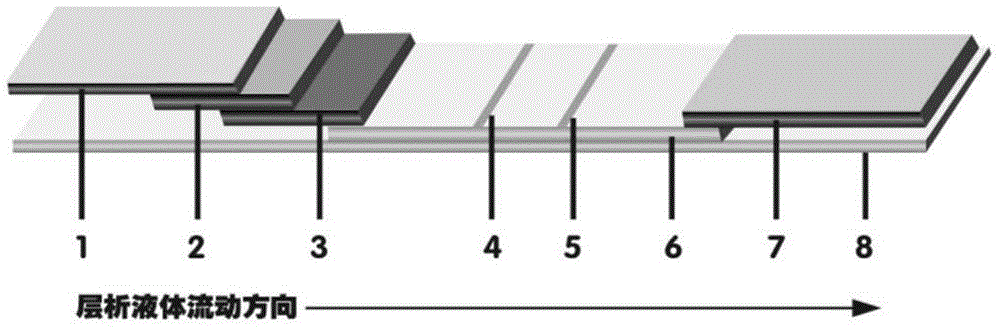
Patents
Literature
38results about How to "Reduce the impact of access" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
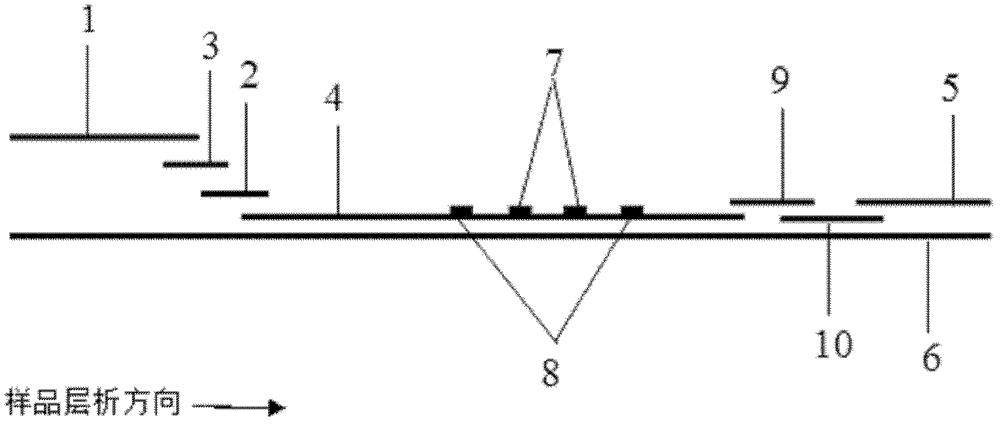
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165ASensitive quantitative detection fastRealize detectionMaterial analysisCritical illnessLinear range
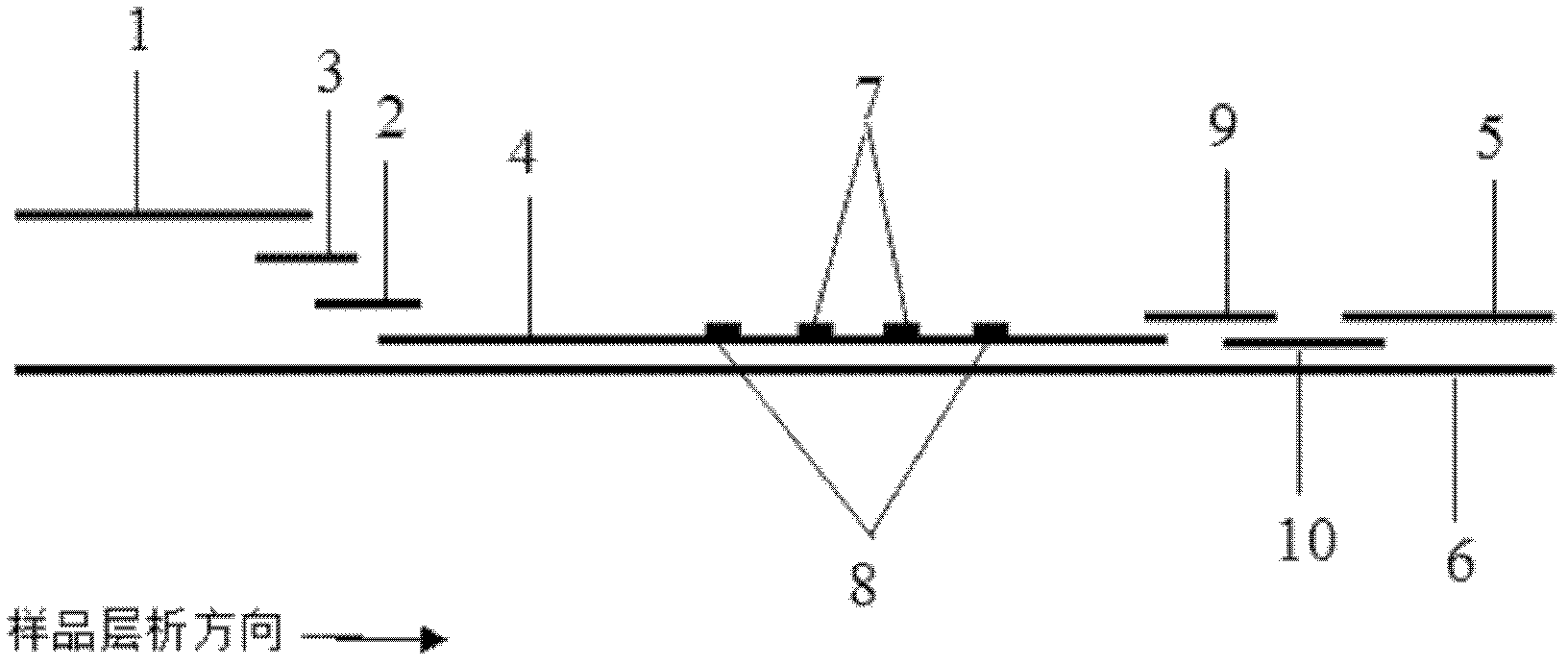
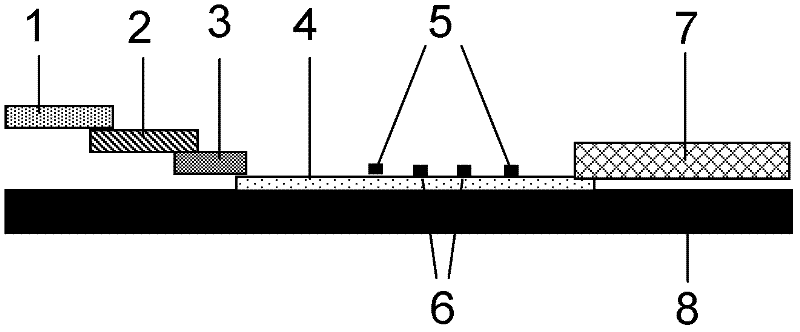
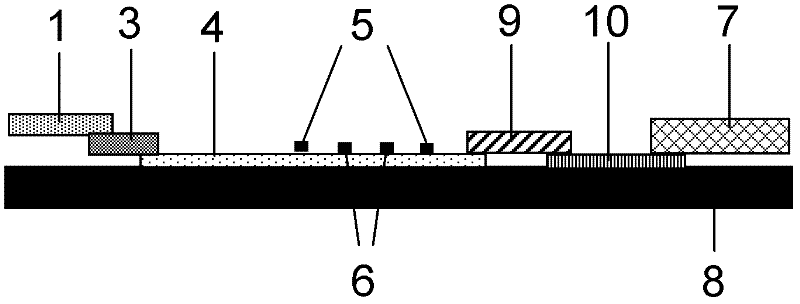
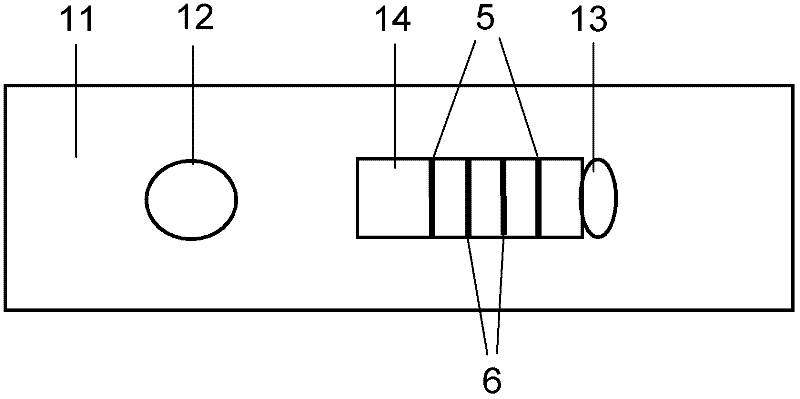
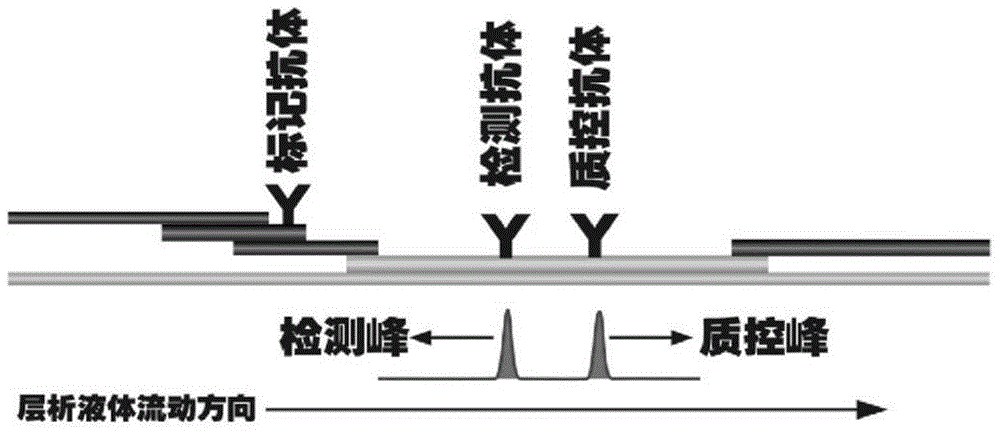
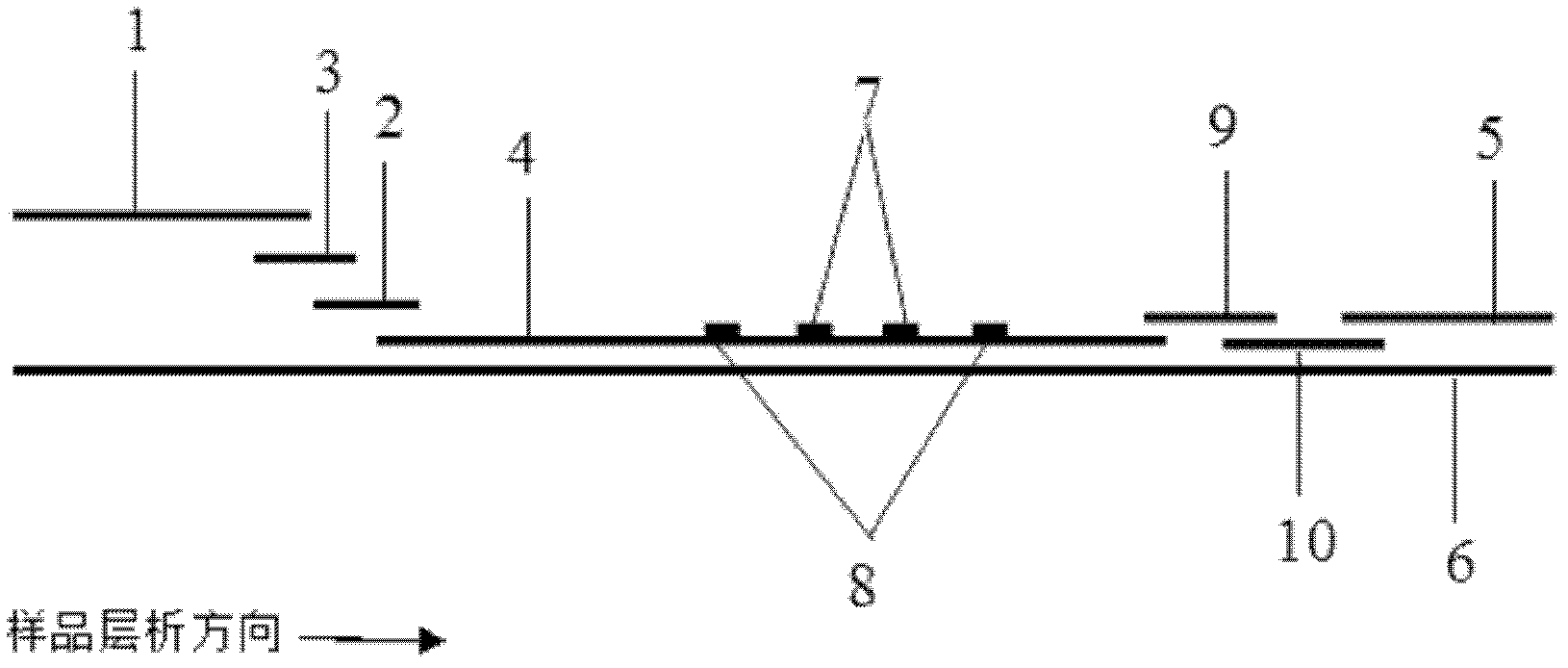
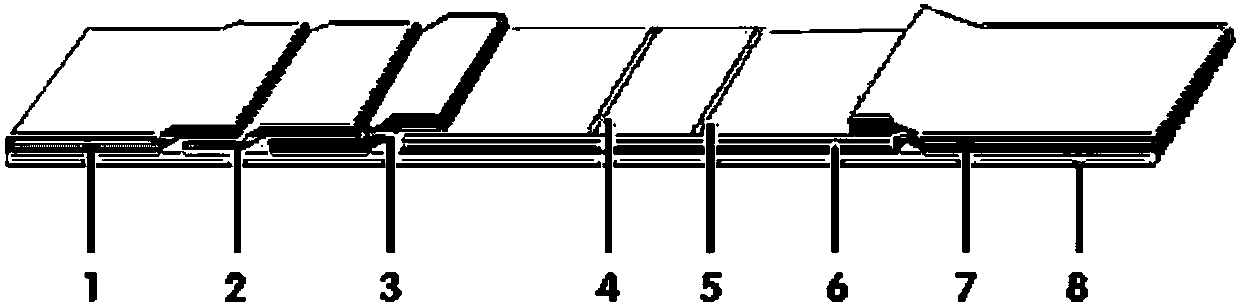
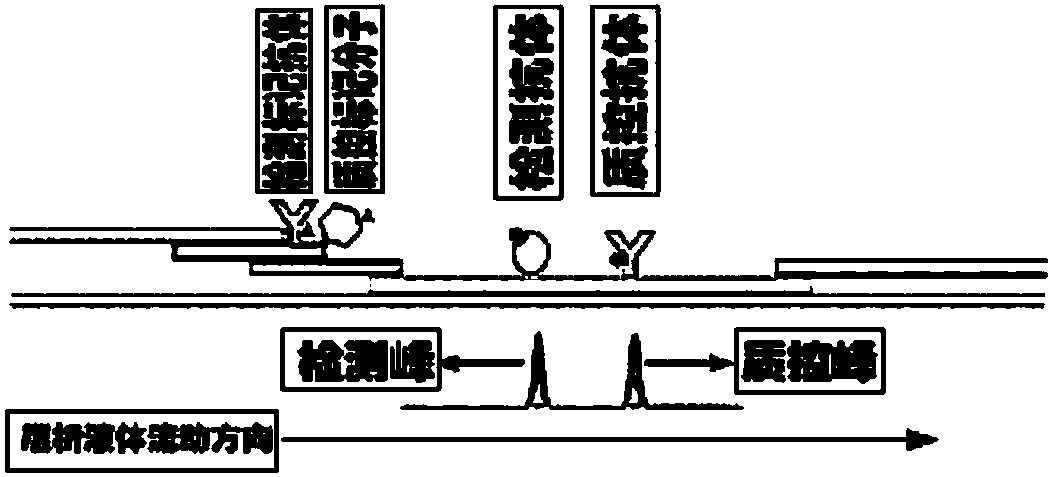
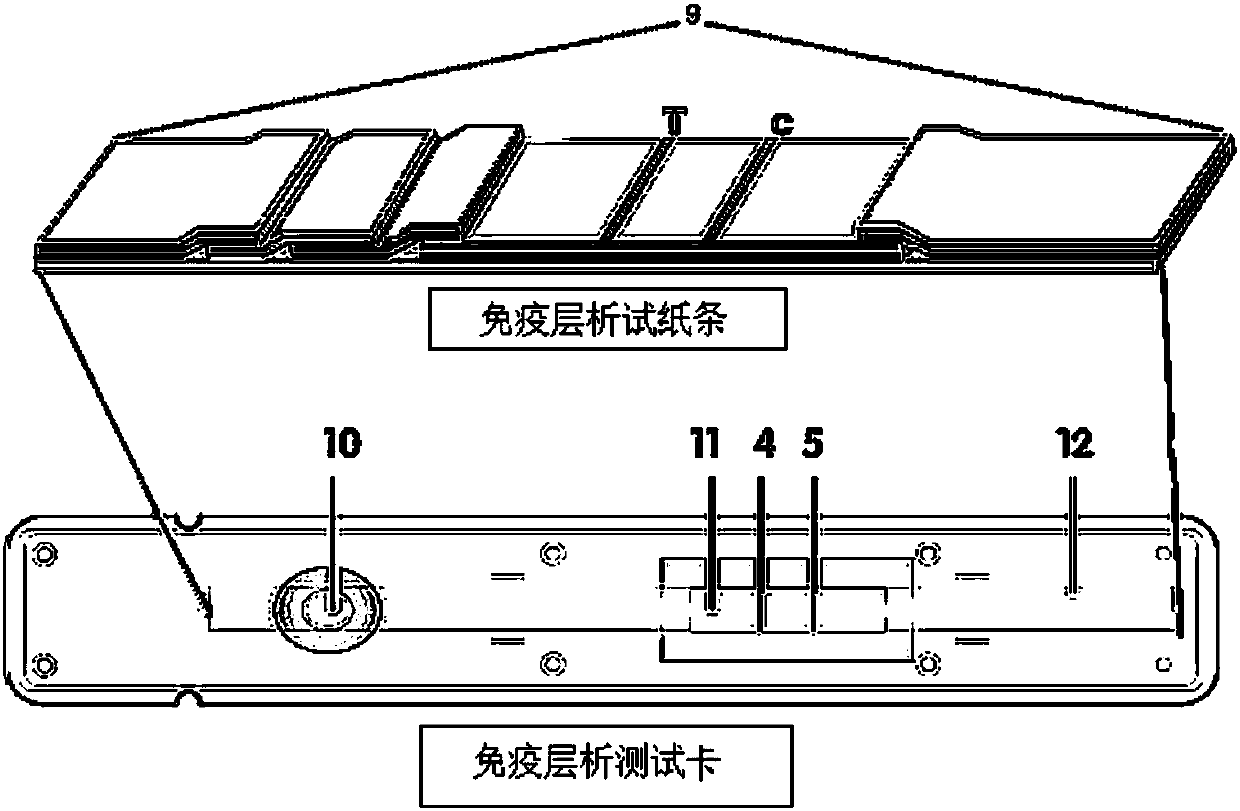
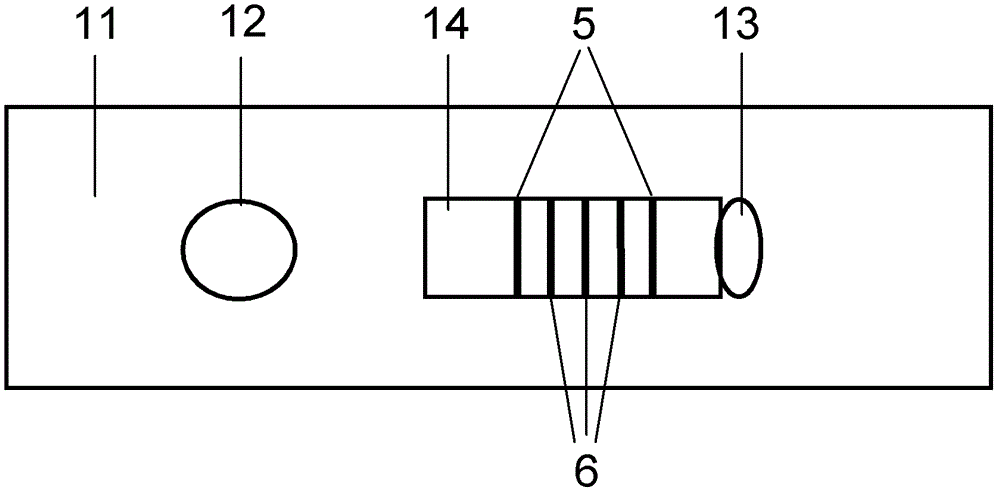
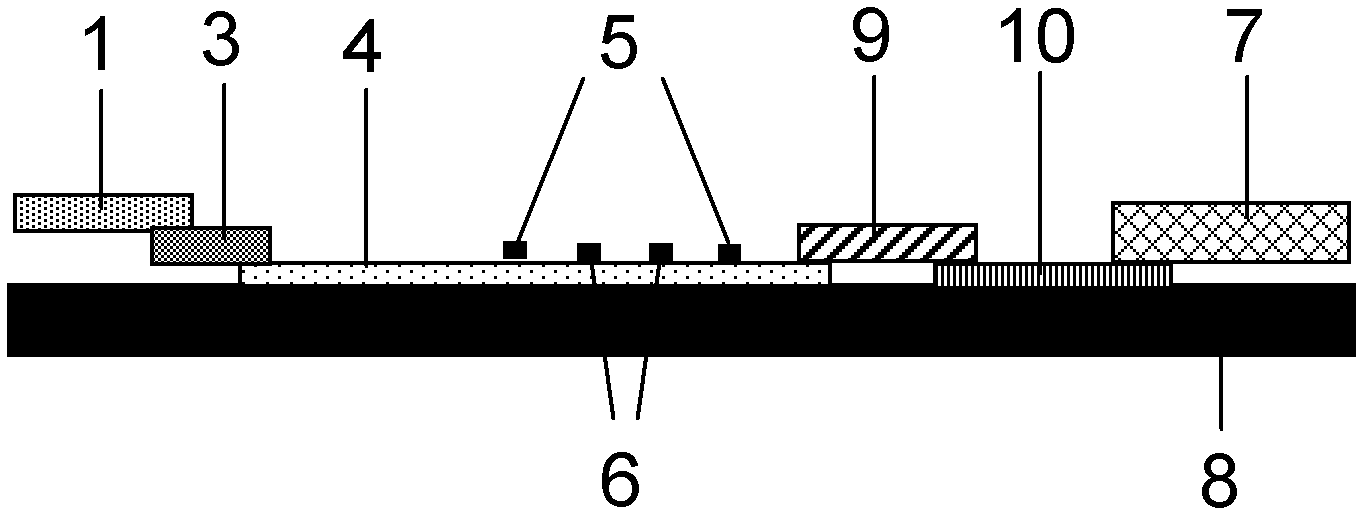
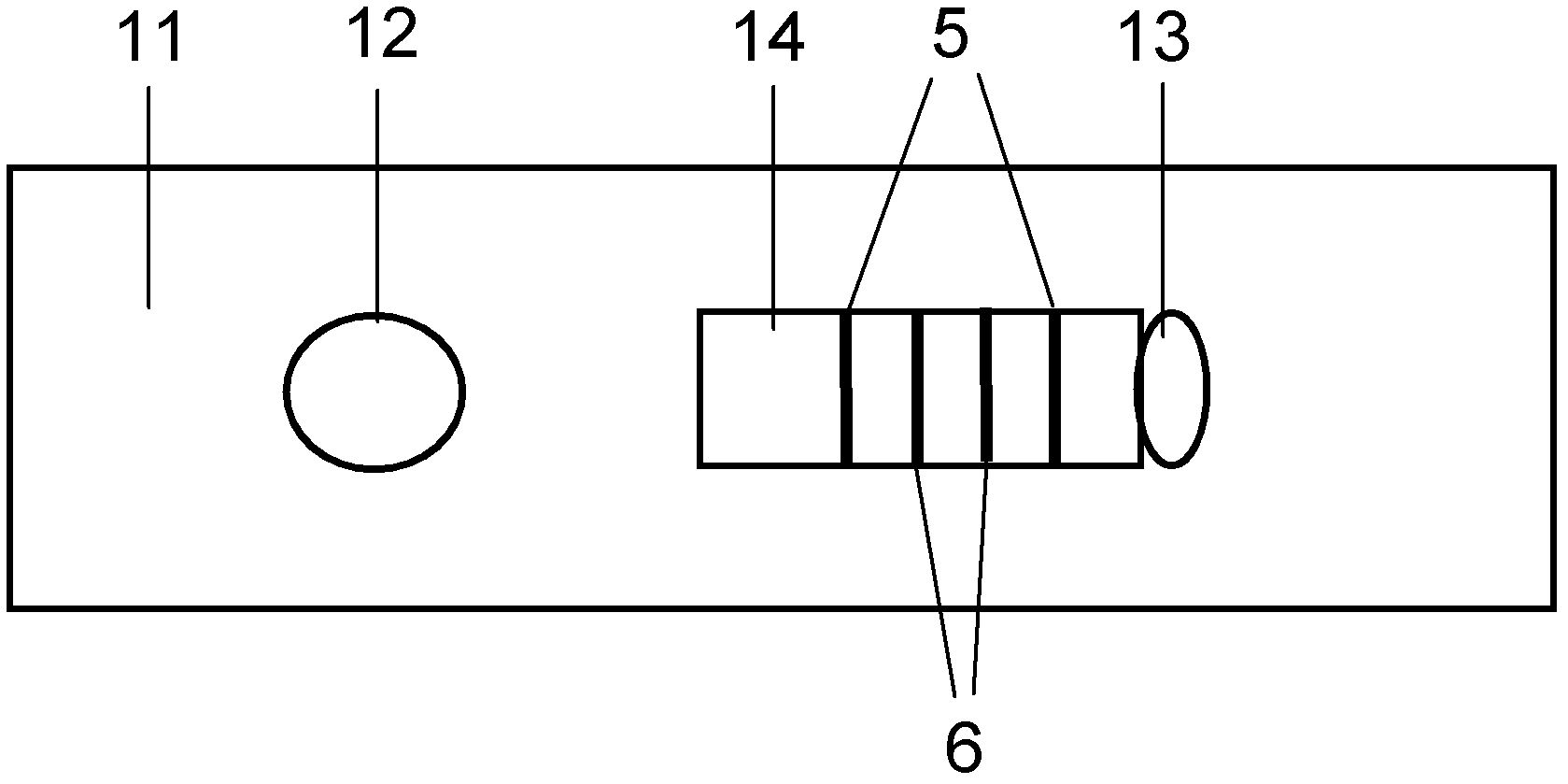
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
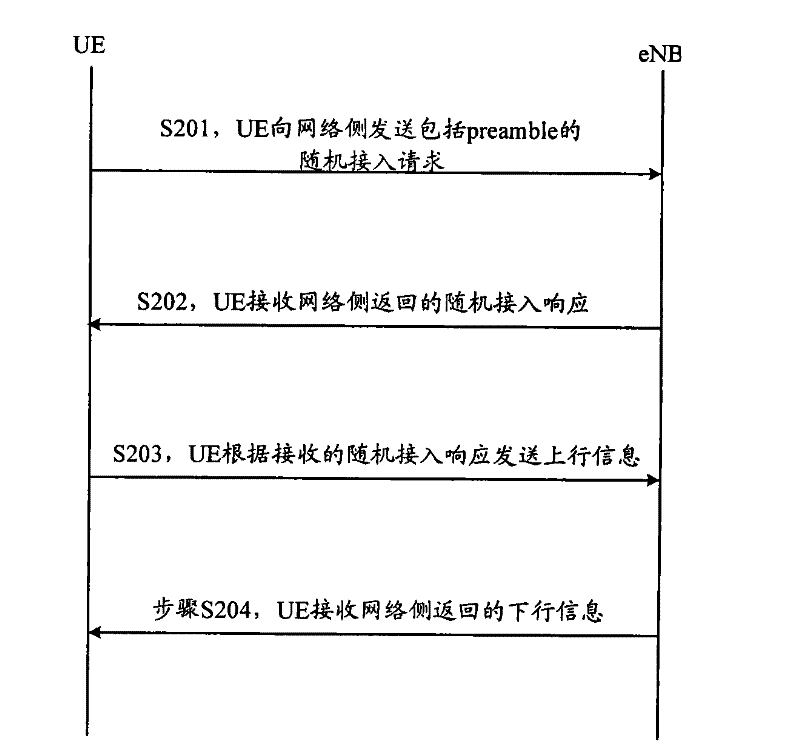
Random access control method of machine type communication (MTC) equipment and MTC equipment
ActiveCN102238752AAvoid congestionReduce the impact of accessError preventionWireless communicationUser equipmentEmbedded system
The invention discloses a random access control method of machine type communication (MTC) equipment and the MTC equipment, and is used for solving the problem caused by random access of multiple pieces of MTC equipment in the current network. The method comprises the following steps of: acquiring relevant parameters of a random access load by monitoring a channel which is used for transmitting a random access response message to other user equipment by a network side; determining the random access load according to the acquired relevant parameters; and controlling random access according to a comparison result between the determined random access load and a set load. In the MTC equipment, the random access is controlled by judging the random access load of the network, the congestion caused by simultaneously accessing multiple MTC terminals to the network can be avoided, the influence of the MTC equipment access on the traditional mobile terminal access is reduced, and the change on the action of network-side equipment is simultaneously reduced.
Owner:DATANG MOBILE COMM EQUIP CO LTD
Fluorescence immunochromatographic assay and kit for quantitative detection of troponin I/creatine kinase isoenzyme/myohemoglobin
ActiveCN102520192AHigh sensitivityHigh detection sensitivityBiological testingNon specificImmunochromatographic Assays
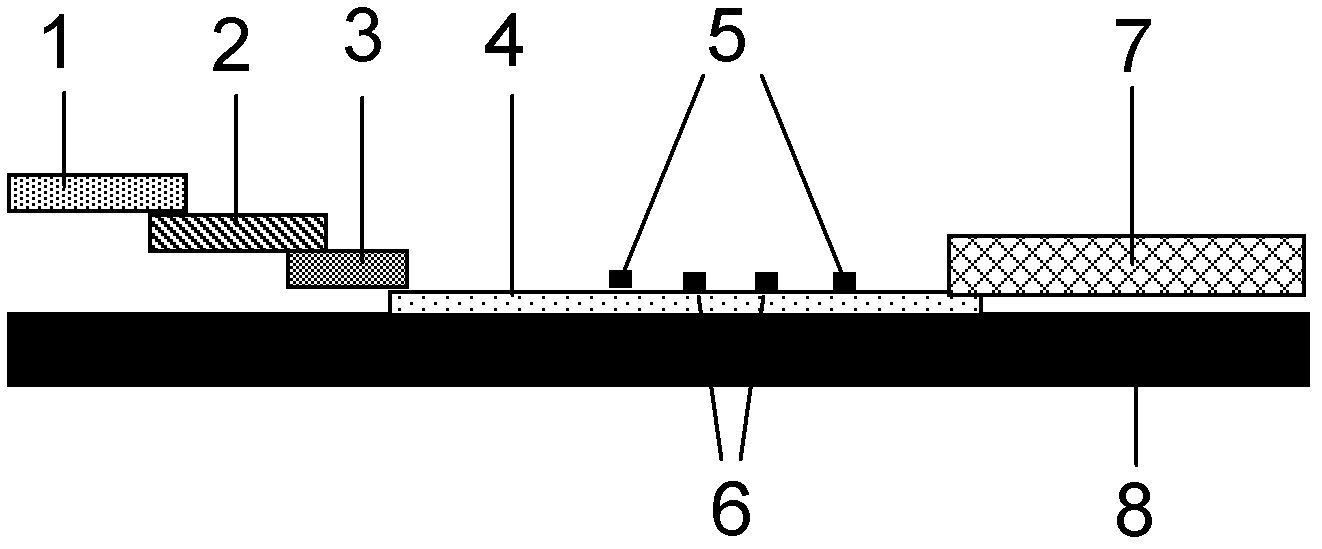
The invention discloses a quantum dot multicolor marking method for quantitative detection of various cardiovascular disease markers and a kit of troponin I / creatine kinase isoenzyme / myohemoglobin. The method realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a multicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the method has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range, small cross interference, and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the troponin I, the creatine kinase isoenzyme and the myohemoglobin simultaneously, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and reagent kit thereof
ActiveCN102539785ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceBasic levelQuantum dot
The invention discloses a fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and a reagent kit thereof. The fluorescent immunochromatography method for the whole quantitative detection of the C-reactive protein (CRP) utilizes excellent fluorescent characteristics of quantum dots, and combines double-color marking technology and immunochromatography technology to achieve fluorescent quantitative detection on the basis of optimizing each constituent elements of test paper. Compared with a conventional colloidal gold immunochromatography method, the fluorescent immunochromatography method for the whole quantitative detection of the CRP has the advantages of being good in stability, low in non-specificity, high in flexibility, wide in linear range and accurate in quantifying. The reagent kit of the fluorescent immunochromatography method can perform the whole quantifying and can simultaneously predict and evaluate infectious diseases, antibiotic effects and cardiovascular and cerebrovascular diseases. The fluorescent immunochromatography method for the whole quantitative detection of the CRP and the reagent kit of the fluorescent immunochromatography method are suitable for various-level hospitals, and particularly contribute to wide popularization in basic-level hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Magnetic fluorescent microsphere immunochromatography quantitative detection method
The invention discloses a magnetic fluorescent microsphere immunochromatography quantitative detection method. In the method, respective excellent characteristics of magnetic nano particles and quantum dots are fully utilized, and an immunochromatography technology is combined to realize fluorescent quantitative detection on the basis of optimizing the structure and ingredients of a test strip. The method has a function of amplifying signals; and compared with the conventional colloidal gold immunochromatography method, the method has the advantages of high mark stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The invention provides a simple, accurate, specific and cheap detection tool for blood samples, urine samples, spittle, excrement and the like, so the method can be widely applied to the fields of medical technology, food safety, veterinary drug residues, environmental monitoring, drug detection and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Fluorescence immunochromatographic assay method for quantitatively detecting heart fatty acid binding protein and kit for quantitatively detecting same
ActiveCN102520194ASolve the backgroundSolve the signal indistinguishableBiological testingBlood plasmaBiology
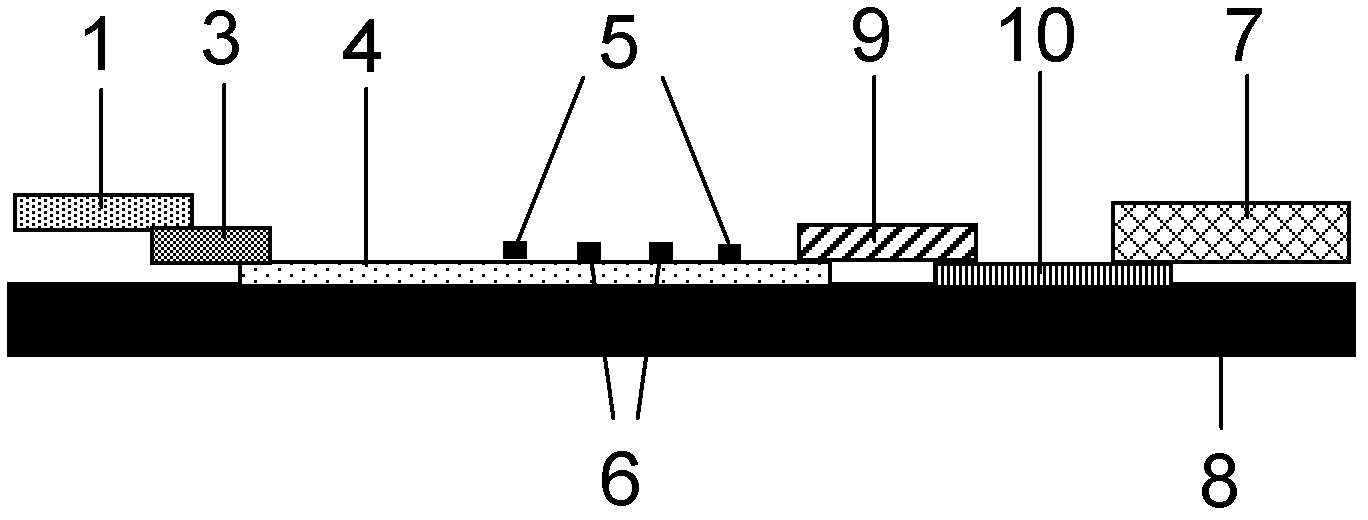
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay and kit for quantitatively detecting N-terminal pro brain natriuretic peptide
ActiveCN102565423ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceDiseaseN-terminal pro-Brain Natriuretic Peptide
Owner:SHENZHEN KANGMEI BIOTECH
Immunofiltration assay fluorescent quantitative detection method based on high-sensitivity quantum dot
ActiveCN102539771AHigh luminous intensityWide excitation spectrumFluorescence/phosphorescenceQuantum dotQuality control
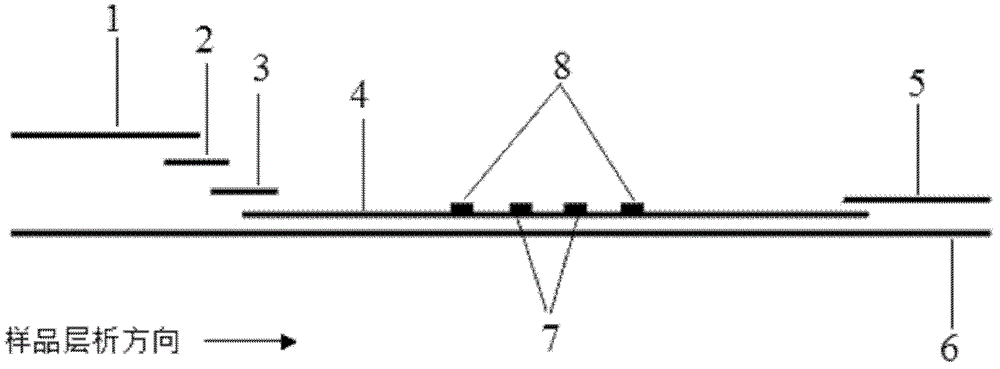
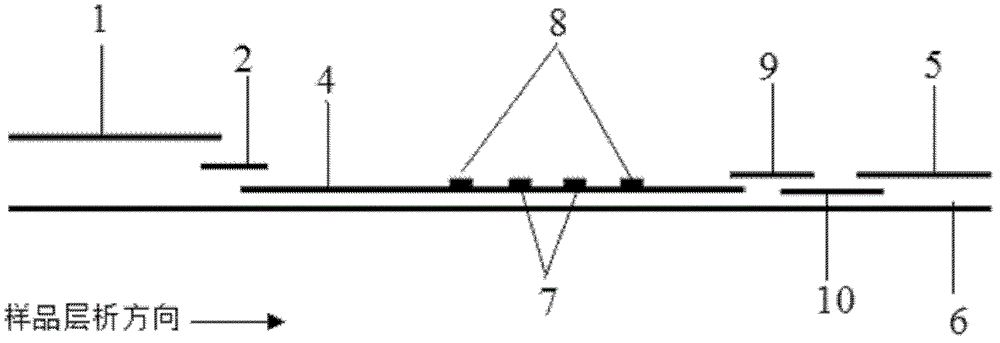
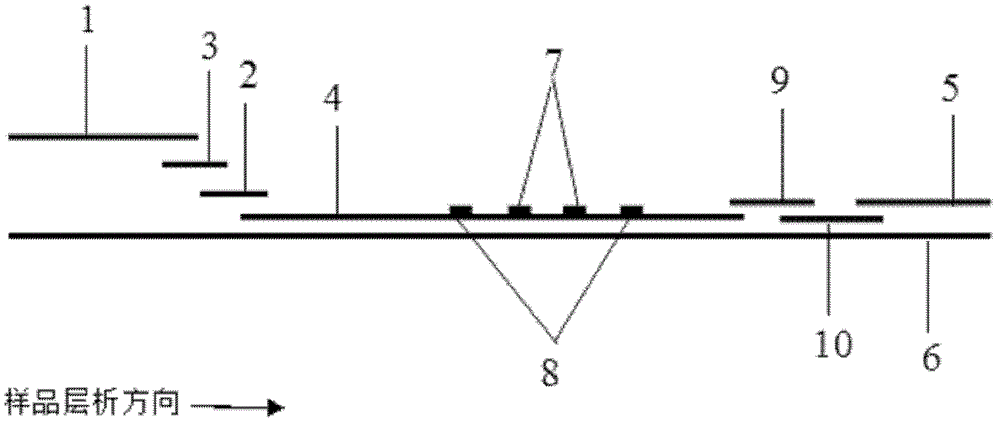
The invention discloses an immunofiltration assay fluorescent quantitative detection method based on a high-sensitivity quantum dot; the immunofiltration assay fluorescent quantitative detection method comprises the following steps of: constructing a fluorescence immunofiltration array device by using the excellent fluorescent characteristic of the quantum dot in combination of a quantum dot fluorescence labeling technology and an immunofiltration array technology on the basis of optimizing constituent parts for the immunofiltration assay; and after immunofiltration array, detecting the strenght of fluorescent signals of the quantum dot and a quality control dot by using a fluorescence quantometer, correcting the fluorescence strenght of the quantum pot by using the quality control dot, and further realizing the quantitative detection of a tested object according to a standard curve obtained by using the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and high in sensitiveness. Comapred with the conventional collodial gold immunofiltration array method, the immunofiltration assay fluorescent quantitative detection method has the advantages of good labeling stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The method is suitable for samples such as serums, urine, spittle, excrement and the like and can be applicable to the detection of serious illness, poisons, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 and preparation method for fluorescence immunochromatography kit
InactiveCN104655858AHigh luminous intensityWide excitation spectrumDisease diagnosisBiological testingPlasma samplesFluorescence
The invention discloses a fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 by taking fluorescent dye as a marker. The fluorescence immunochromatography kit disclosed by the invention realizes fluorescence quantitative detection for the human epididymis secretory protein-4, has the advantages of being good in stability, wide in linear range, good in specificity, accurate to quantify, simple and quick, can be used for simultaneously detecting whole blood, blood serum and plasma samples, and is suitable for hospitals of various levels.
Owner:DEMAIJI BIOTECH BEIJING
Fluorescence immunochromatographic assay and kit for quantitative detection of human cardiac troponin I (cTnI)
ActiveCN102520193ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of cardiac troponin I (cTnI). The fluorescence immunochromatographic assay for quantitative detection of the cTnI realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the cTnI, can be used for detecting whole blood, blood serum and plasma samples simultaneously, and is applied to different levels of hospitals and is particularly favored to be widely popularized to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay and kit for quantitative detection of creatine kinase isoenzyme (CK-MB)
ActiveCN102520173ASolve the backgroundSolve the signal indistinguishableMaterial analysisDiseaseCreatine kinase
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of acute myocardial infarction marker-creatine kinase isoenzyme (CK-MB). The fluorescence immunochromatographic assay for quantitative detection of the CK-MB realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the CK-MB, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Two-photon fluorescence immunochromatography kit for quantitative determination of anti-Mullerian hormone (AMH) and preparation method of kit
InactiveCN107621540ALong fluorescence lifetimeHigh luminous intensityMaterial analysisQuantitative accuracyBlood plasma
The invention discloses a two-photon fluorescence immunochromatography kit for quantitative determination of anti-Mullerian hormone (AMH), which utilizes fluorescent dye as a maker. The two-photon fluorescence immunochromatography kit, realizing fluorescence immunochromatography quantitative determination, has the advantages of good stability, wide linear range, good specificity, high sensitivity,high quantitative accuracy and easy and quick operation, can be applied to detection of whole blood samples, serum samples and plasma samples simultaneously, and is applicable to medical treatment ofhospital at different levels and family practice.
Owner:DEMAIJI BIOTECH BEIJING
Low-orbit satellite Internet of Things channel resource dynamic allocation method based on SDN
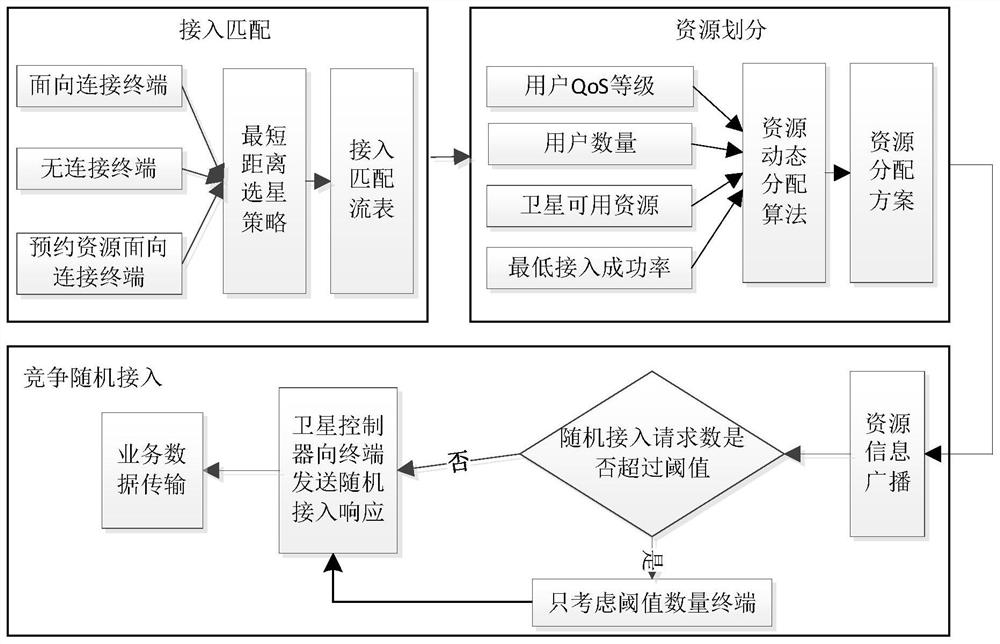
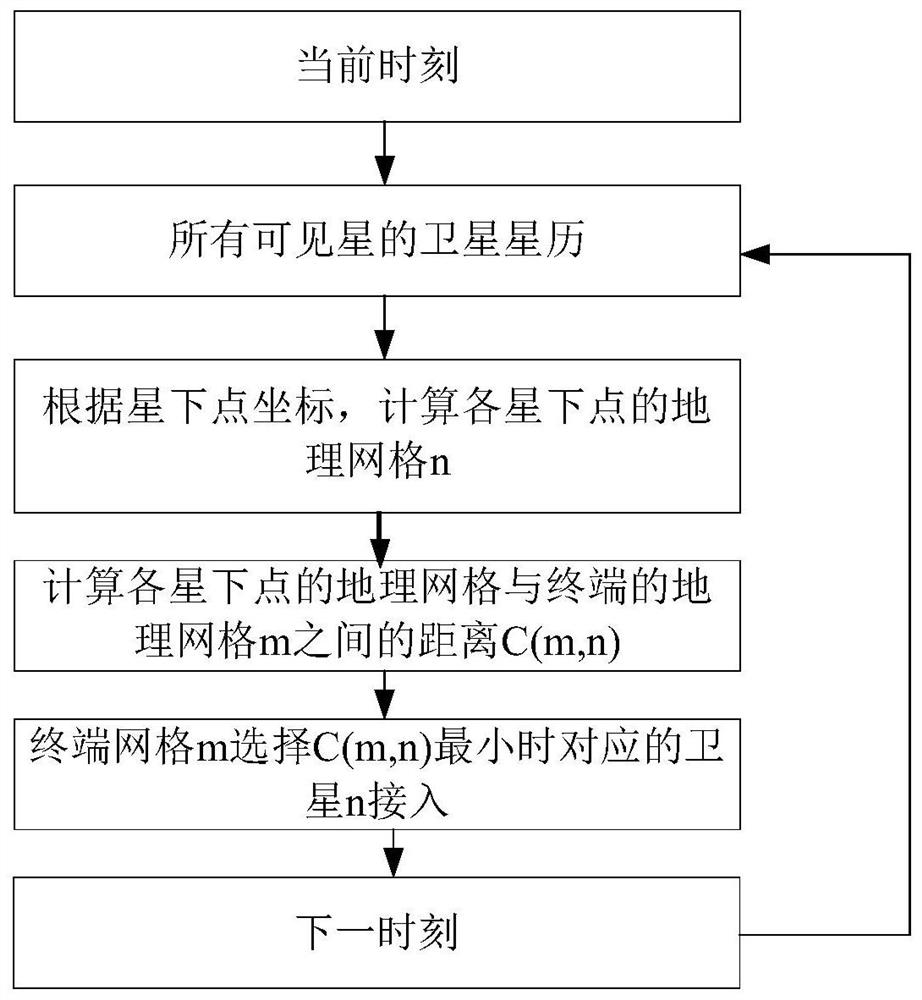
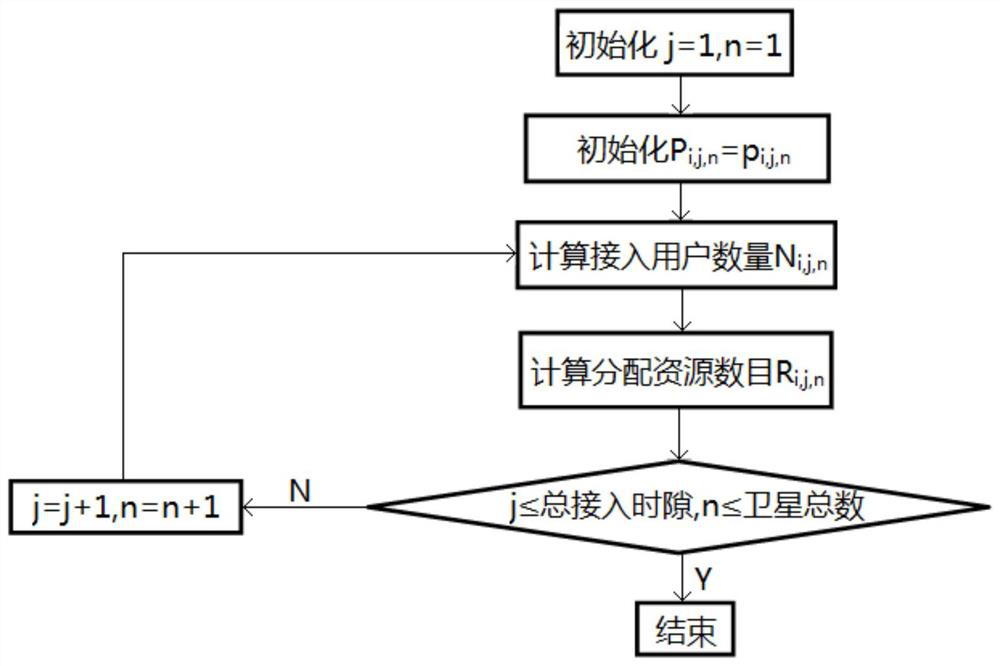
ActiveCN112272412ASolve problems such as centralized management difficultiesMaximize access success rateNetwork topologiesTelecommunicationsGeoweb
The invention provides a low-orbit satellite Internet of Things channel resource dynamic allocation method based on SDN. In an access matching flow table calculation step, the distance between the sub-satellite point position of each satellite and the geographic grid where a satellite Internet of Things terminal is located is calculated through a ground controller to judge which satellite should be selected by the terminal for access; in a satellite channel resource dynamic allocation step, the QoS grade, the quantity, the minimum access success rate limit and the satellite available resourcesof the access terminal service are calculated through a satellite controller, and the channel resources are dynamically divided; and in an access control assisted random access step, competitive random access is carried out according to the channel resources allocated by the terminal. According to the method, on the basis of an SDN architecture, channel resources are allocated through cooperativeoptimization of the ground controller and the satellite controller, so that the access success rate of high-priority services is maximized and the access performance of services with different priorities is guaranteed while the success rate of accessing channels of services with medium and low priorities is not lower than a specified threshold value.
Owner:NANJING UNIV OF POSTS & TELECOMM
Network attack processing method and device
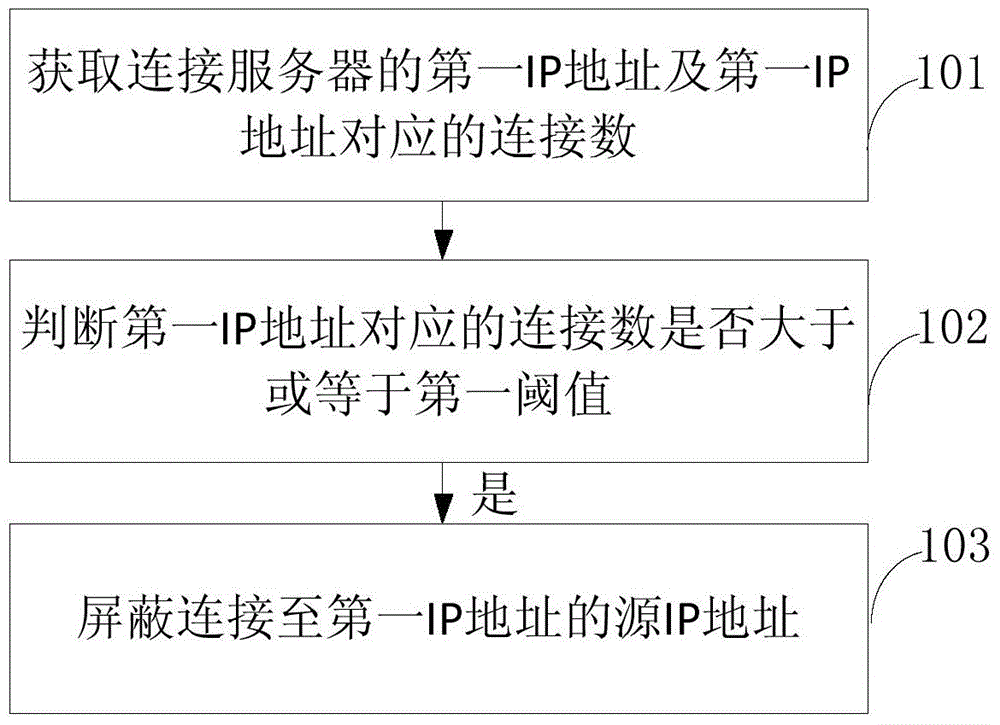
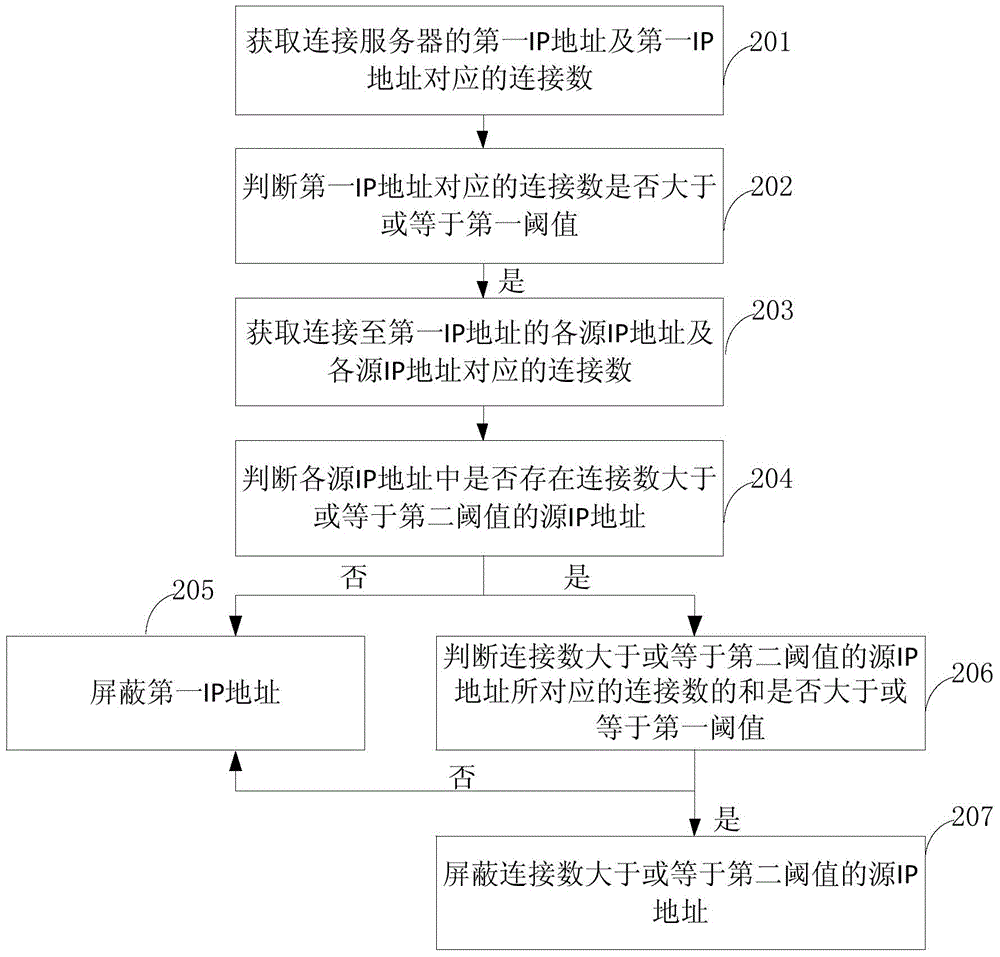
ActiveCN106302347AReduce business impactReduce the impact of accessSecuring communicationIp addressConnection number
The invention provides a network attack processing method and device The network attack processing method comprises steps of obtaining a first IP address connected to a server and a connection number corresponding to the first IP address, determining whether the connection number corresponding to the first IP address is greater than or equal to a first threshold value, if yes, shielding a source IP address connected to the first IP address. The embodiment of the network attack processing method and device not only guarantees integral stability of the server and the network, but also reduces influence on a service of an attacked IP address through shielding the source IP address, and reduces the influence on the network access.
Owner:ALIBABA GRP HLDG LTD
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165BHigh luminous intensityWide excitation spectrumMaterial analysisCritical illnessUrine sample
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Magnetic fluorescent microsphere immunochromatography quantitative detection method
ActiveCN102565386BReduce sample matrix varianceHigh detection sensitivityFluorescence/phosphorescenceAdditive ingredientMedical testing
The invention discloses a magnetic fluorescent microsphere immunochromatography quantitative detection method. In the method, respective excellent characteristics of magnetic nano particles and quantum dots are fully utilized, and an immunochromatography technology is combined to realize fluorescent quantitative detection on the basis of optimizing the structure and ingredients of a test strip. The method has a function of amplifying signals; and compared with the conventional colloidal gold immunochromatography method, the method has the advantages of high mark stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The invention provides a simple, accurate, specific and cheap detection tool for blood samples, urine samples, spittle, excrement and the like, so the method can be widely applied to the fields of medical technology, food safety, veterinary drug residues, environmental monitoring, drug detection and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Fluorescence immunochromatographic assay and kit for quantitative detection of troponin I/creatine kinase isoenzyme/myohemoglobin
ActiveCN102520192BHigh luminous intensityWide excitation spectrumBiological testingCreatine kinaseFluorescence
The invention discloses a quantum dot multicolor marking method for quantitative detection of various cardiovascular disease markers and a kit of troponin I / creatine kinase isoenzyme / myohemoglobin. The method realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a multicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the method has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range, small cross interference, and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the troponin I, the creatine kinase isoenzyme and the myohemoglobin simultaneously, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay and kit for quantitative detection of human cardiac troponin I (cTnI)
ActiveCN102520193BHigh luminous intensityWide excitation spectrumBiological testingFluorescenceQuantum dot
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of cardiac troponin I (cTnI). The fluorescence immunochromatographic assay for quantitative detection of the cTnI realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the cTnI, can be used for detecting whole blood, blood serum and plasma samples simultaneously, and is applied to different levels of hospitals and is particularly favored to be widely popularized to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay kit for quantitatively detecting heart fatty acid binding protein
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Immunofiltration assay fluorescent quantitative detection method based on high-sensitivity quantum dot
ActiveCN102539771BHigh luminous intensityWide excitation spectrumFluorescence/phosphorescenceQuantum dotQuality control
The invention discloses an immunofiltration assay fluorescent quantitative detection method based on a high-sensitivity quantum dot; the immunofiltration assay fluorescent quantitative detection method comprises the following steps of: constructing a fluorescence immunofiltration array device by using the excellent fluorescent characteristic of the quantum dot in combination of a quantum dot fluorescence labeling technology and an immunofiltration array technology on the basis of optimizing constituent parts for the immunofiltration assay; and after immunofiltration array, detecting the strenght of fluorescent signals of the quantum dot and a quality control dot by using a fluorescence quantometer, correcting the fluorescence strenght of the quantum pot by using the quality control dot, and further realizing the quantitative detection of a tested object according to a standard curve obtained by using the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and high in sensitiveness. Comapred with the conventional collodial gold immunofiltration array method, the immunofiltration assay fluorescent quantitative detection method has the advantages of good labeling stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The method is suitable for samples such as serums, urine, spittle, excrement and the like and can be applicable to the detection of serious illness, poisons, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
System for quantitatively detecting heavy metal cadmium and preparation method thereof
PendingCN111198264AHigh luminous intensityWide excitation spectrumMaterial analysisSample dilutionHeavy metals
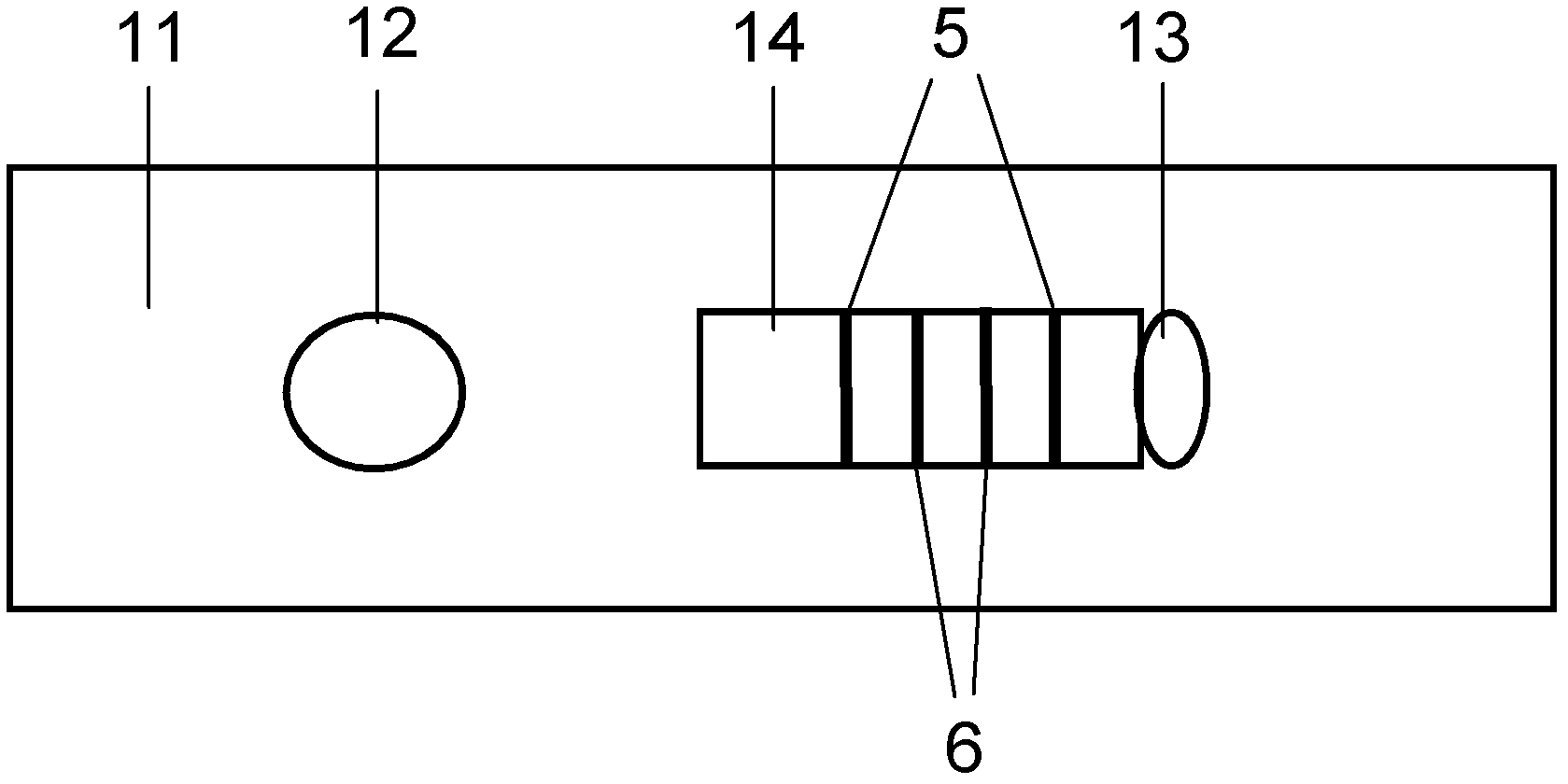
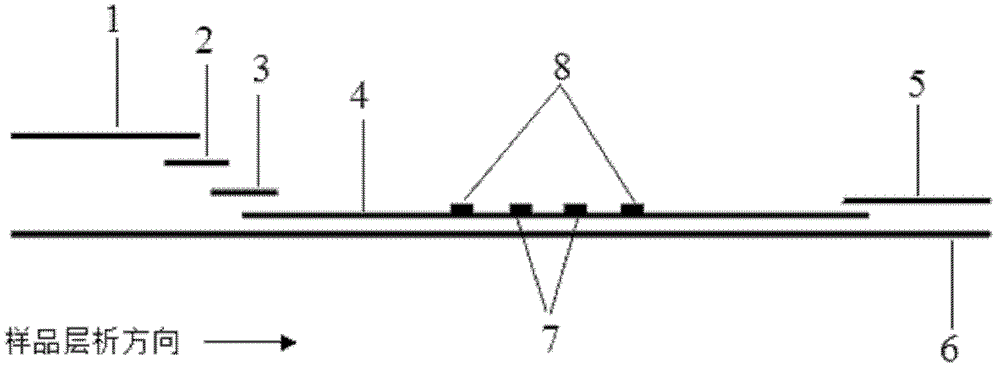
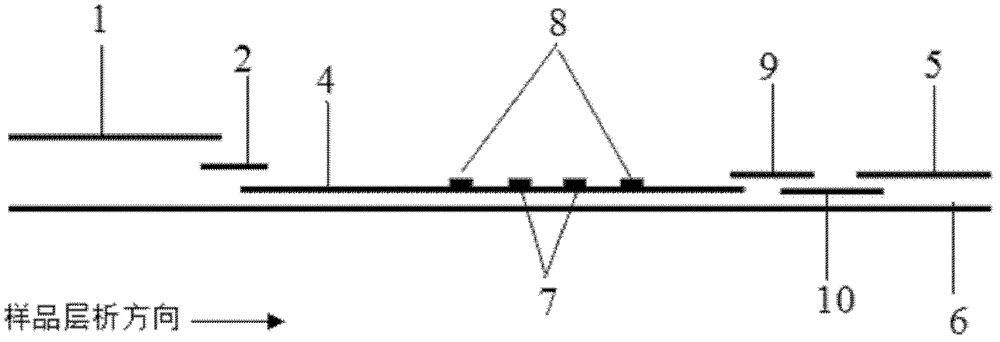
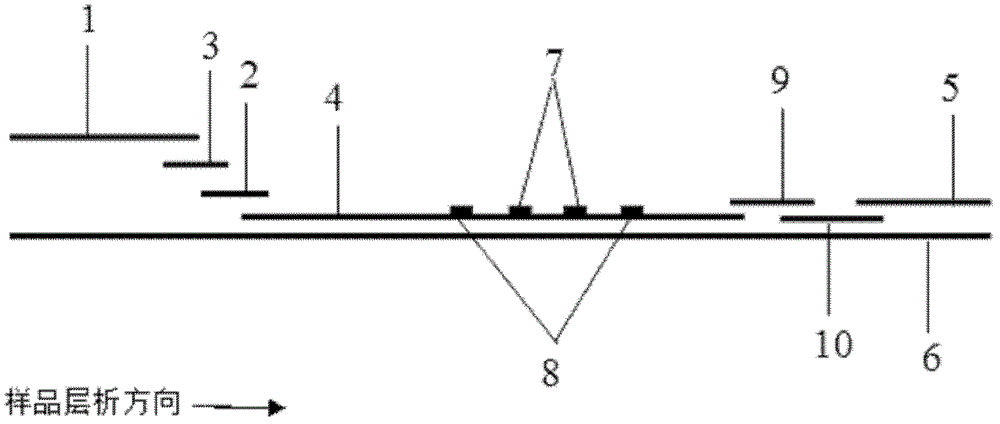
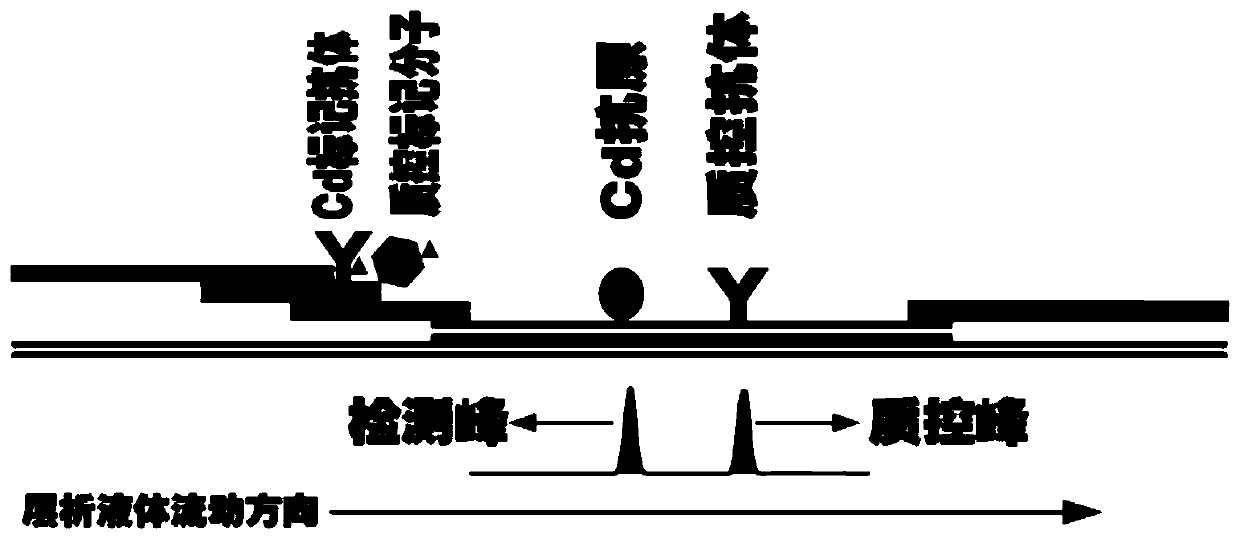
The invention provides a system for quantitatively detecting heavy metal cadmium, which comprises a buckle, a fluorescence immunochromatography test strip and a sample dilution buffer solution, wherein the buckle is of an outer shell structure of the fluorescence immunochromatography test strip and is provided with a sample adding hole and an observation window; the fluorescence immunochromatography test strip comprises a sample pad, a marking pad, a chromatography membrane, a water absorption pad and a bottom plate; a fluorescent dye modified heavy metal cadmium specific antibody and a fluorescent dye modified quality control molecule are fixed on the marking pad at the same time; a heavy metal cadmium chelating agent hapten is fixed on the quantitative detection line; the hapten is specifically combined with a fluorescent dye modified heavy metal cadmium specific antibody fixed on the marking pad; biomolecules capable of being specifically combined with the quality control moleculesare fixed on the quality control line; and a to-be-detected sample is treated with the sample dilution buffer solution before detection. In order to detect the heavy metal cadmium in the sample, treatment by using the sample dilution buffer solution is carried out before the to-be-detected sample is detected, and the sample dilution comprises a chelating agent which is combined with the heavy metal cadmium to form a heavy metal cadmium chelating agent hapten; if the sample dilution buffer solution is not used for treating a sample, the sample is directly detected, the heavy metal cadmium in the sample cannot be detected, and the existence of the sample dilution buffer solution is crucial to the detection of the cadmium in the sample.
Owner:北京大弘生物技术有限公司
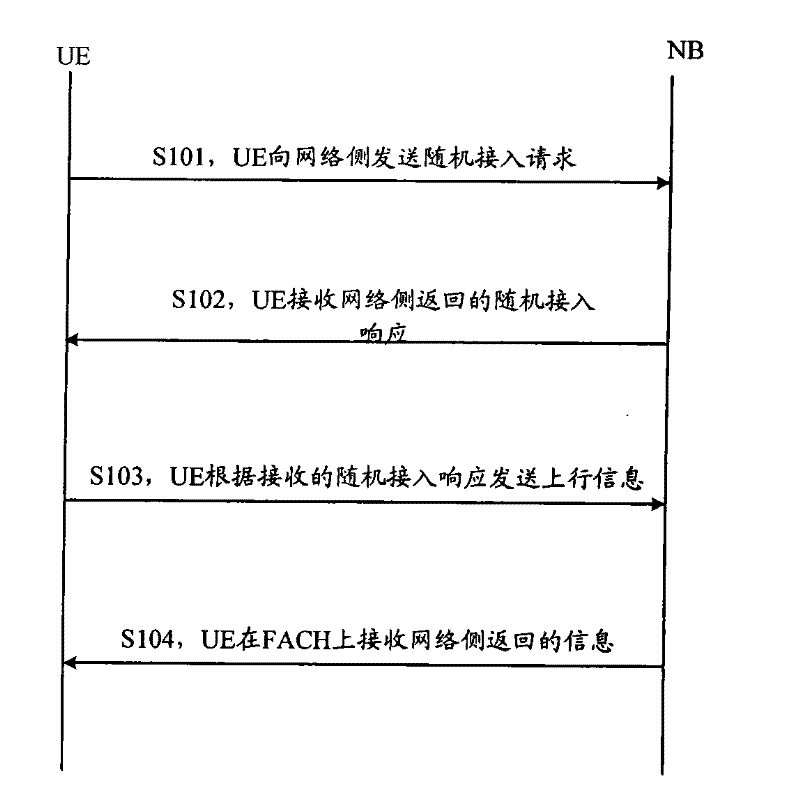
Detection method and apparatus
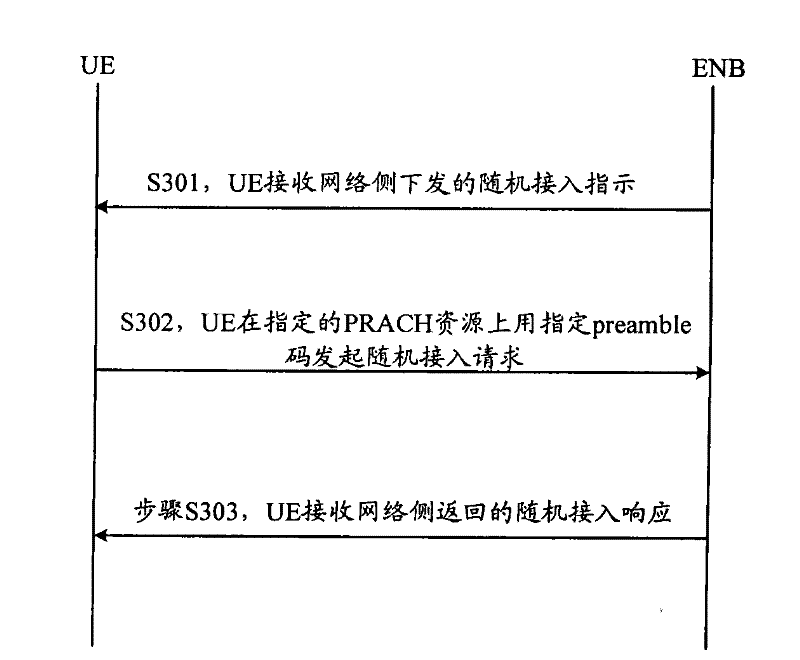
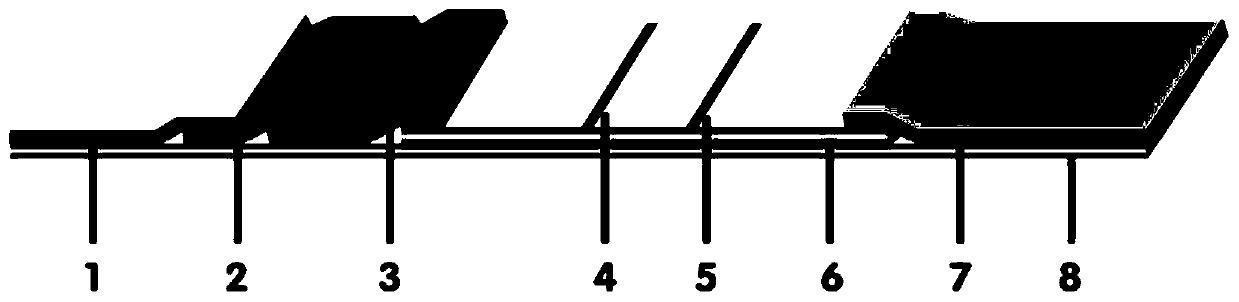
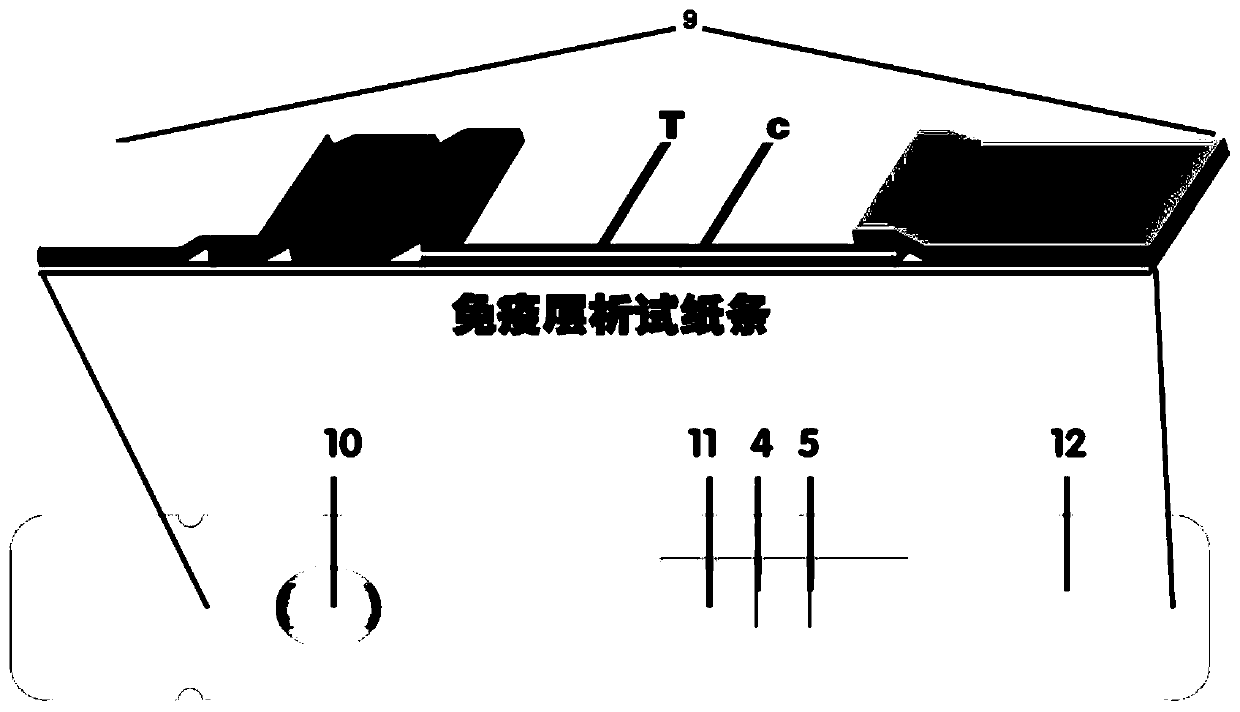
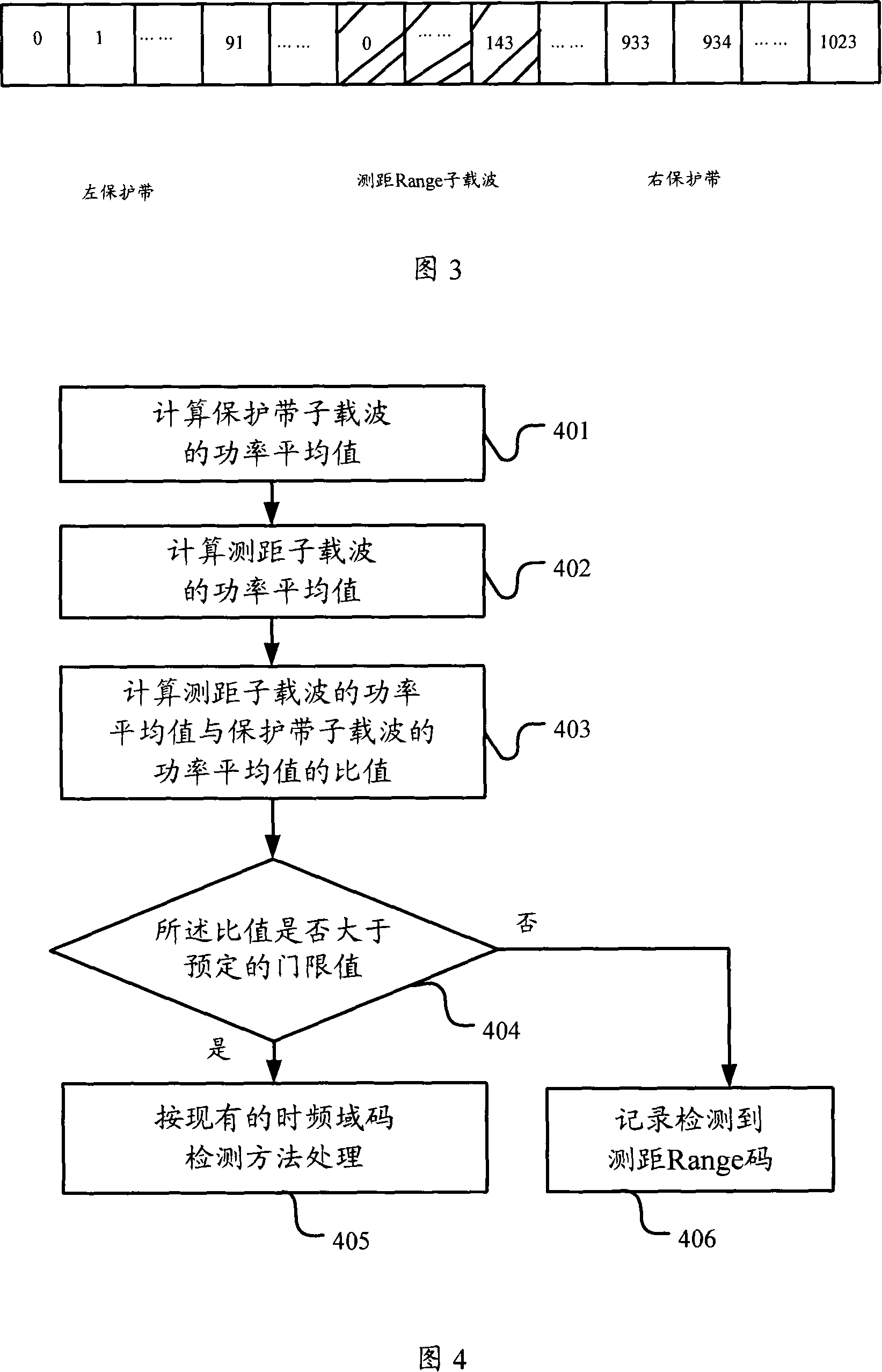
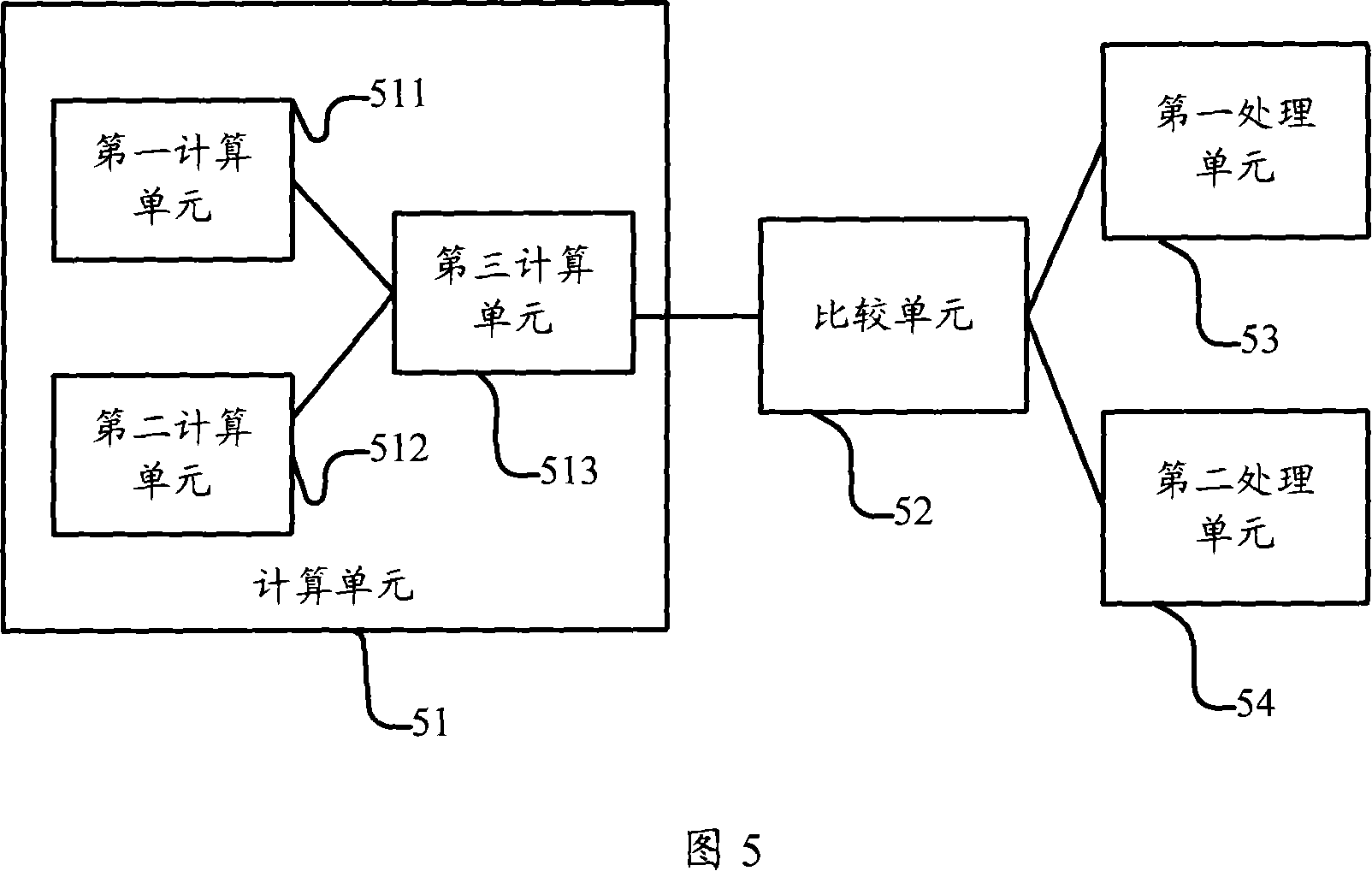
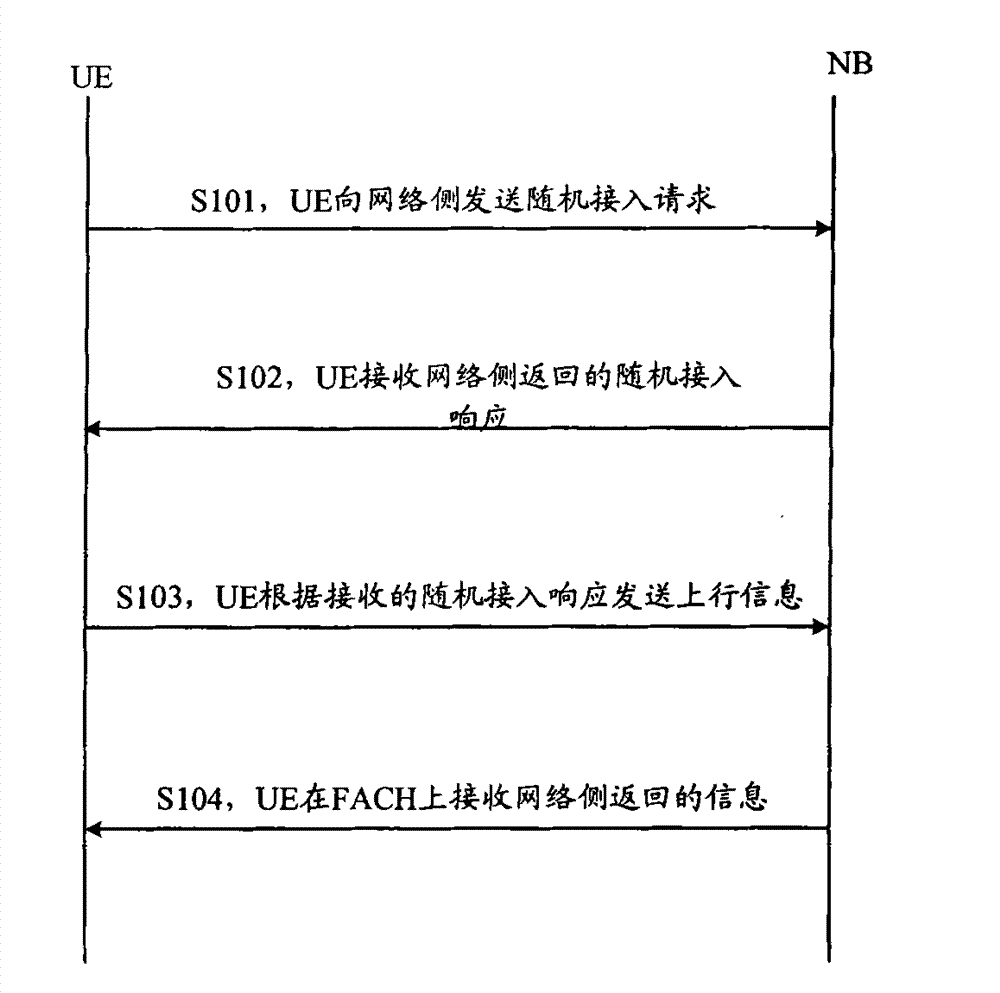
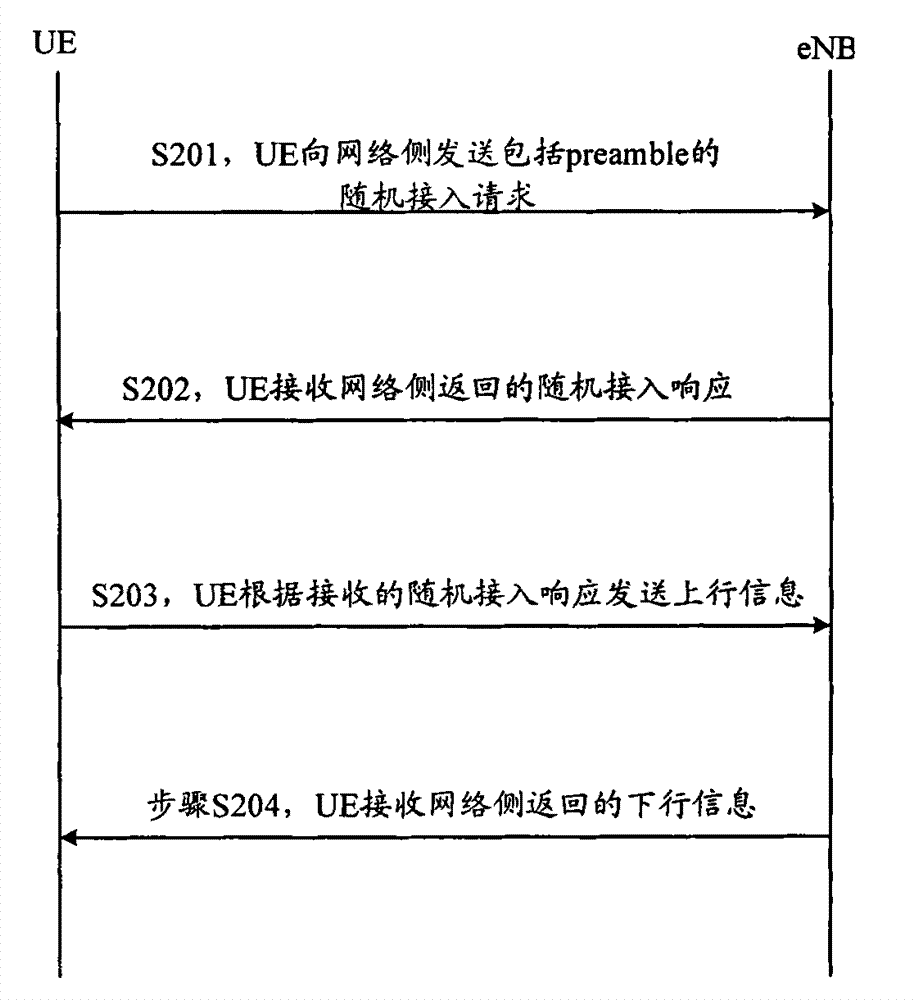
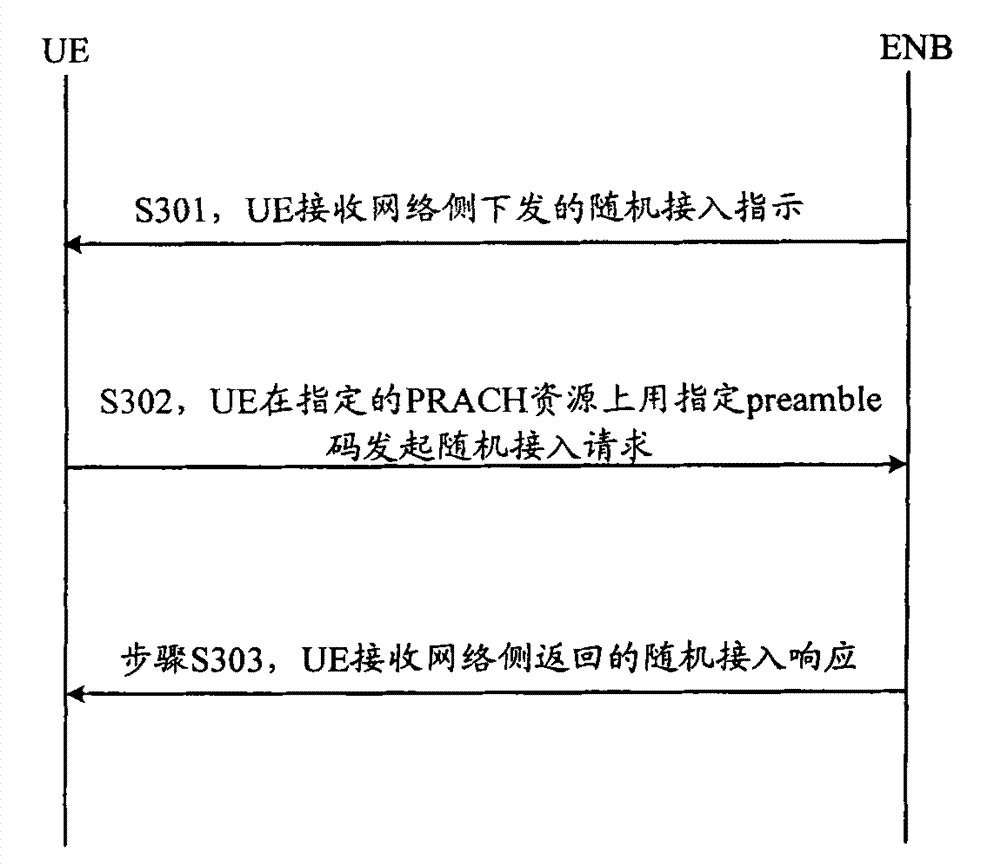
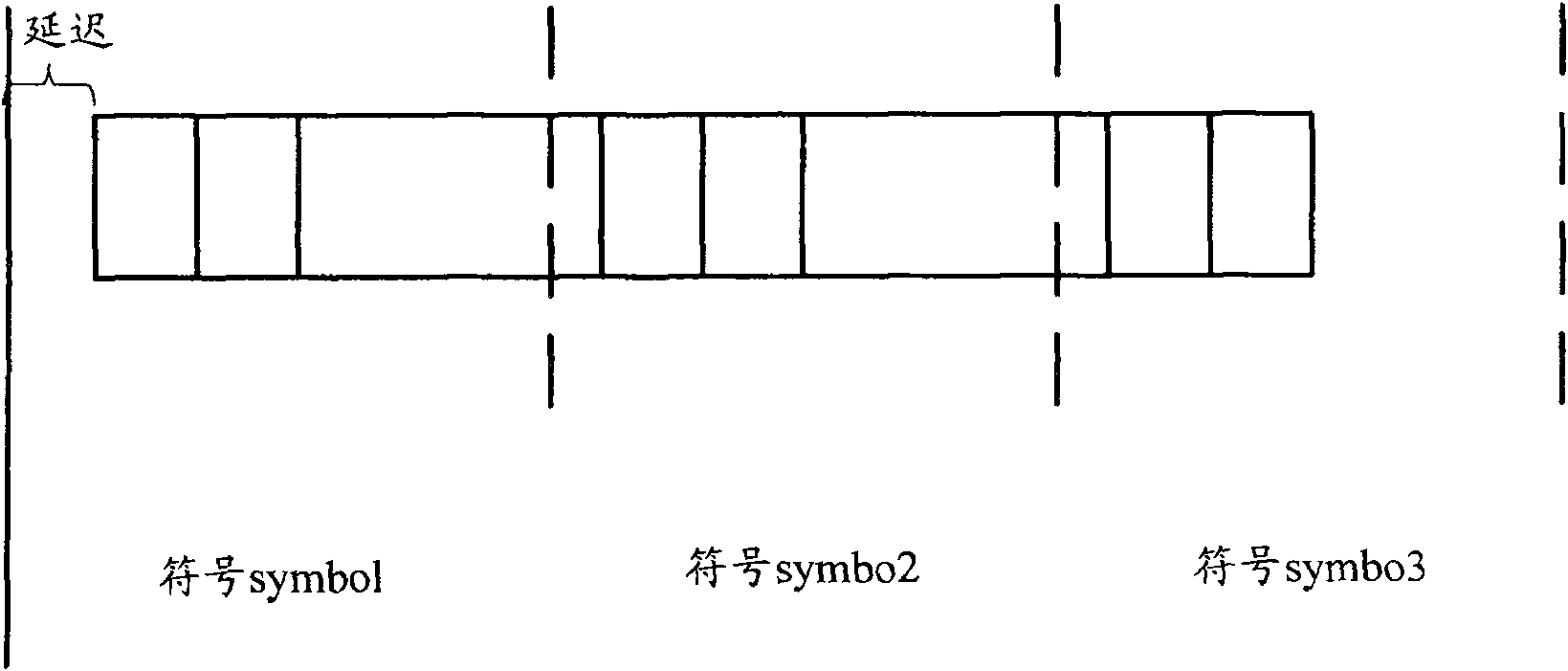
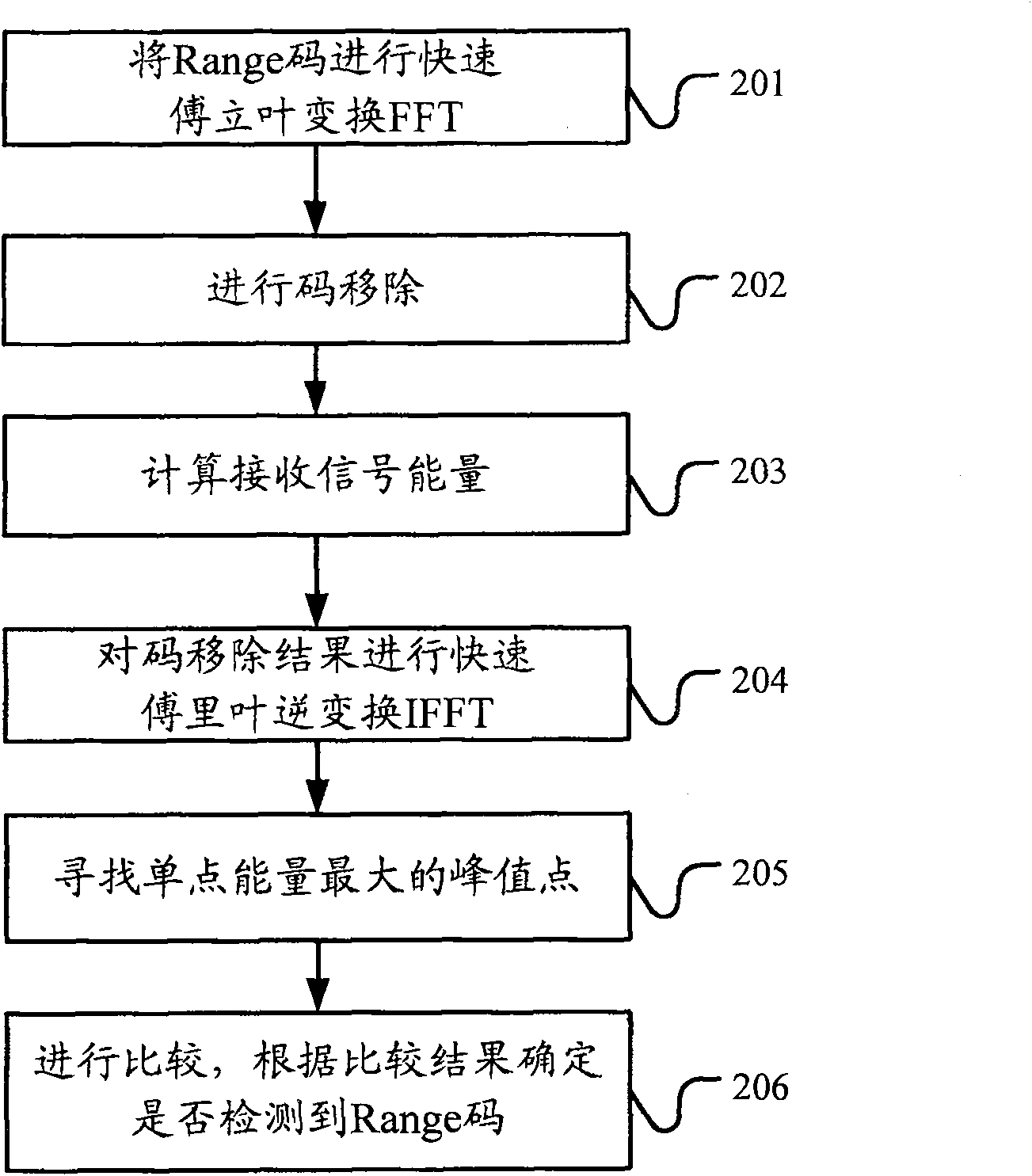
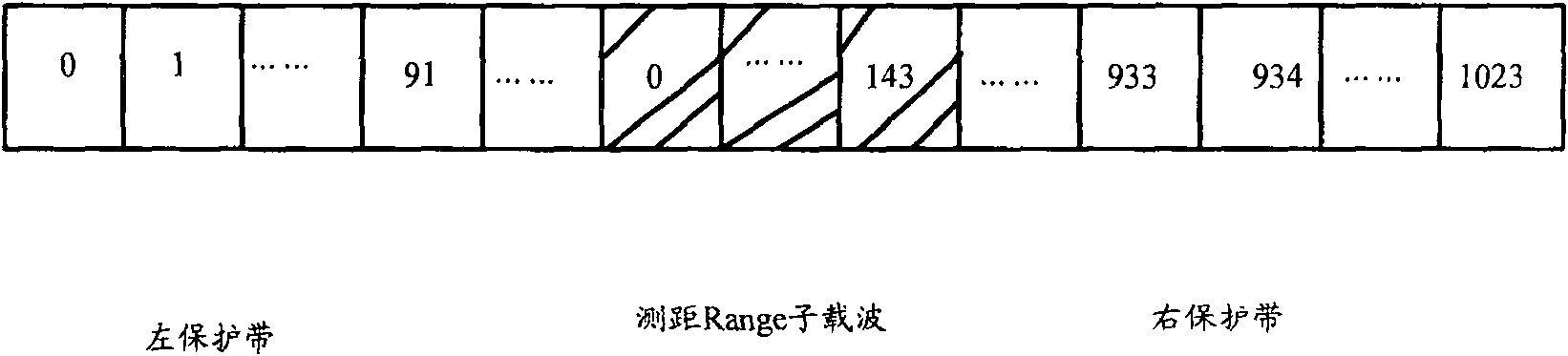
ActiveCN101083840AReduce the impact of accessReduce the impactRadio/inductive link selection arrangementsMulti-frequency code systemsCarrier signalEngineering
The present invention discloses a detection method, it includes as follows. Calculating the ratio of the average power of ranging sub-carriers and the average power of protection zone carrier; comparing the above mentioned ratio with the scheduled threshold, and recording the ranging-code If it is less than or equal to the threshold; Accordingly, the implementation of this invention would provide a detection device, including: Computational modules, which could be used to calculate ratio of the average power of ranging sub-carriers and the average power of protection zone carrier; Comparison module, which could be used to compare the referred ratio to the predetermined threshold; The first processing unit, which could be used to record the detected ranging-codes, if the comparison results of the comparison unit is that described ratio is less than or equal to the threshold. Implementation of this invention could provide better technical solutions to the eliminate false alarms, and reduce the impact of user terminal access.
Owner:XFUSION DIGITAL TECH CO LTD
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422BHigh luminous intensityWide excitation spectrumBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Random access control method of machine type communication (MTC) equipment and MTC equipment
ActiveCN102238752BAvoid congestionReduce the impact of accessError preventionWireless communicationUser equipmentEmbedded system
The invention discloses a random access control method of machine type communication (MTC) equipment and the MTC equipment, and is used for solving the problem caused by random access of multiple pieces of MTC equipment in the current network. The method comprises the following steps of: acquiring relevant parameters of a random access load by monitoring a channel which is used for transmitting a random access response message to other user equipment by a network side; determining the random access load according to the acquired relevant parameters; and controlling random access according to a comparison result between the determined random access load and a set load. In the MTC equipment, the random access is controlled by judging the random access load of the network, the congestion caused by simultaneously accessing multiple MTC terminals to the network can be avoided, the influence of the MTC equipment access on the traditional mobile terminal access is reduced, and the change on the action of network-side equipment is simultaneously reduced.
Owner:DATANG MOBILE COMM EQUIP CO LTD
Detection method and apparatus
ActiveCN100551130CReduce the impact of accessEliminate false alarmsRadio/inductive link selection arrangementsMulti-frequency code systemsCarrier signalFalse alarm
The invention discloses a detection method, comprising: calculating the ratio of the power average value of the ranging subcarrier to the power average value of the guard band subcarrier; comparing the ratio with a predetermined threshold value, and if the , then record the detected ranging code. Correspondingly, an embodiment of the present invention provides a detection device, including: a calculation unit, configured to calculate the ratio of the average power value of the ranging subcarrier to the average power value of the guard band subcarrier; a comparison unit, configured to compare the ratio with the The predetermined threshold value is compared; the first processing unit is used to record the detection of the ranging code when the comparison result of the comparison unit is that the ratio is less than or equal to the threshold value. The technical solutions provided by the embodiments of the present invention can better eliminate false alarms, and at the same time reduce the impact on user terminal access.
Owner:XFUSION DIGITAL TECH CO LTD
Fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and reagent kit thereof
ActiveCN102539785BReduce sensitivityHigh luminous intensityBiological testingFluorescence/phosphorescenceFluorescenceAntibiotic effect
The invention discloses a fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and a reagent kit thereof. The fluorescent immunochromatography method for the whole quantitative detection of the C-reactive protein (CRP) utilizes excellent fluorescent characteristics of quantum dots, and combines double-color marking technology and immunochromatography technology to achieve fluorescent quantitative detection on the basis of optimizing each constituent elements of test paper. Compared with a conventional colloidal gold immunochromatography method, the fluorescent immunochromatography method for the whole quantitative detection of the CRP has the advantages of being good in stability, low in non-specificity, high in flexibility, wide in linear range and accurate in quantifying. The reagent kit of the fluorescent immunochromatography method can perform the whole quantifying and can simultaneously predict and evaluate infectious diseases, antibiotic effects and cardiovascular and cerebrovascular diseases. The fluorescent immunochromatography method for the whole quantitative detection of the CRP and the reagent kit of the fluorescent immunochromatography method are suitable for various-level hospitals, and particularly contribute to wide popularization in basic-level hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
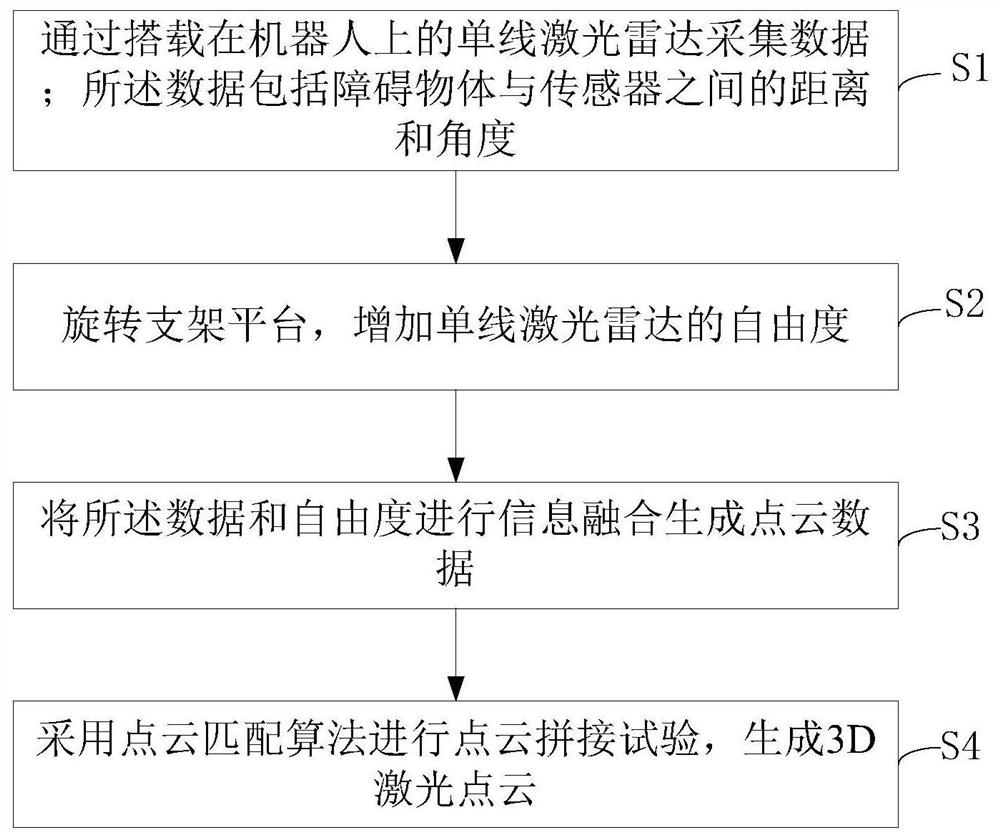
A method for obtaining 3D laser point cloud based on single-line lidar
ActiveCN111580129BIncrease freedomRotation cumulative error eliminationElectromagnetic wave reradiationData packPoint cloud
The invention discloses a method for acquiring 3D laser point clouds based on a single-line laser radar, comprising: S1, collecting data through a single-line laser radar mounted on a robot; the data includes distances and angles between obstacles and sensors; S2 , rotate the bracket platform to increase the degree of freedom of the single-line lidar; the single-line lidar is mounted on the bracket platform, and the bracket platform is mounted on the robot; S3, the data and the degree of freedom are fused to generate point cloud data; S4, using The point cloud matching algorithm is used for point cloud splicing experiments to generate 3D laser point clouds. The present invention adopts a single-line laser radar, which can spin the single-line laser radar around the x-axis of the sensor coordinate system while reducing the use of other sensors, increasing the degree of freedom of the single-line laser radar, obtaining 3D laser point cloud data, and performing point cloud splicing experiment.
Owner:SOUTH CHINA UNIV OF TECH
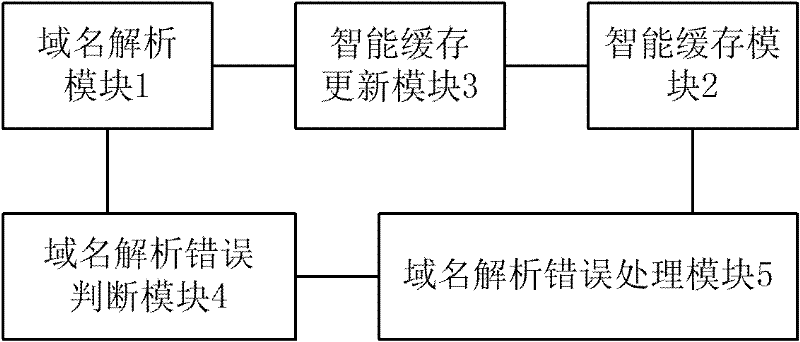
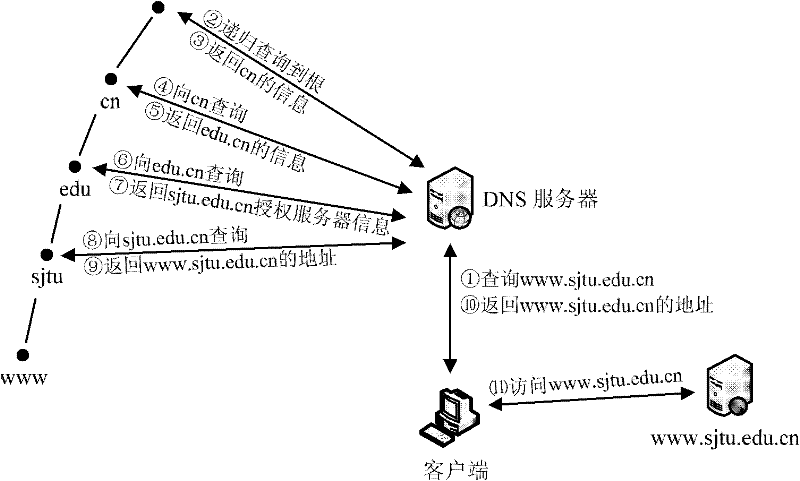
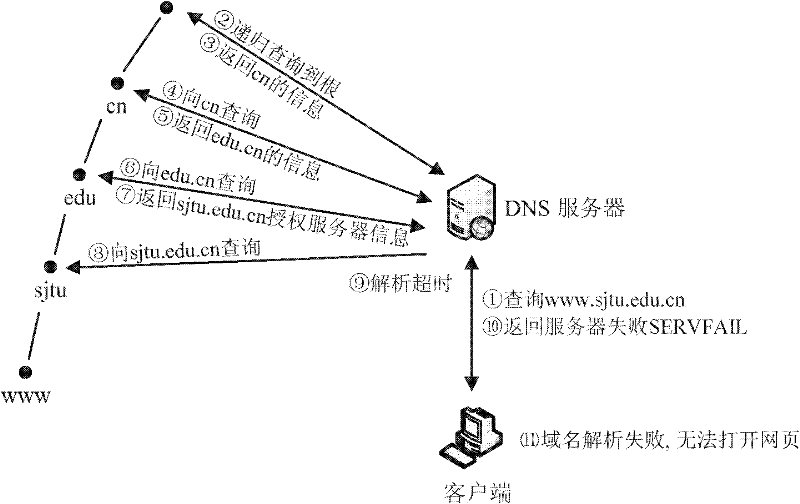
Domain name resolution service system with intelligent buffer and service method thereof
InactiveCN101815105BImprove reliabilityReduce the impact of accessTransmissionError processingProcess module
Owner:SHANGHAI JIAOTONG UNIV
Fluorescence immunochromatographic assay and kit for quantitatively detecting N-terminal pro brain natriuretic peptide
ActiveCN102565423BHigh luminous intensityWide excitation spectrumBiological testingFluorescence/phosphorescenceDiseaseN-terminal pro-Brain Natriuretic Peptide
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting acute myocardial infarction marker, namely, N-terminal pro brain natriuretic peptide (NT-proBNP). The fluorescence immunochromatographic assay for quantitatively detecting the NT-proBNP realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the NT-proBNP and detecting whole blood, blood serum and blood plasma samples, can provide reference for diagnosis of cardiovascular and cerebrovascular diseases and can be widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com