Fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and reagent kit thereof
A technology of fluorescent immunochromatography and reactive protein, which is applied in the field of fluorescent immunochromatographic method and its kit for quantitative detection of C-reactive protein in the whole process, which can solve the problems of low sensitivity and narrow detection range of C-reactive protein, and achieve the goal of expanding Effects of improved detection range, improved quantitative accuracy, and improved sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
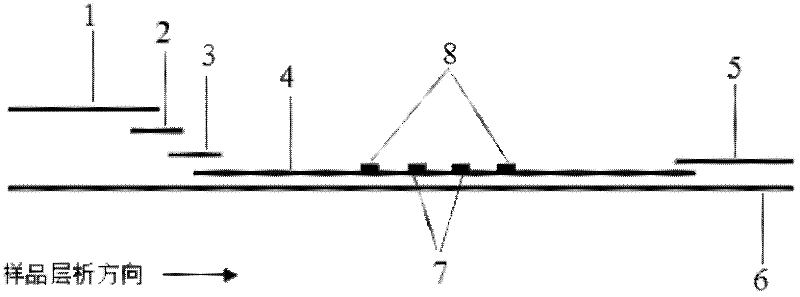
[0072] Example 1: Quantitative detection of CRP by modifying antibodies to quantum dots by chemical cross-linking and using direct pre-wetting immunochromatography
[0073] (1) Modification of quantum dots and antibodies
[0074] Quantum dots with an emission wavelength of 650nm were mixed with 1mg / mL CRP monoclonal antibody, and mixed with freshly prepared N-hydroxysuccinimide (NHS, 1mg / mL) and carbodiimide hydrochloride (EDC, 1mg / mL) under the action of room temperature for 4 to 5 hours, adding 1mol / L glycine to block, and using a chromatographic column or chromatographic column to separate and purify to obtain CRP monoclonal antibody modified quantum dots. Similarly, rabbit IgG-modified quantum dots were obtained. The fluorescence emission wavelength of the CRP antibody-modified quantum dot is 650nm, and the fluorescence emission wavelength of the rabbit IgG-modified quantum dot is 570nm.
[0075] (2) Construction of the kit
[0076] Mix the two kinds of quantum dot mar...
Embodiment 2
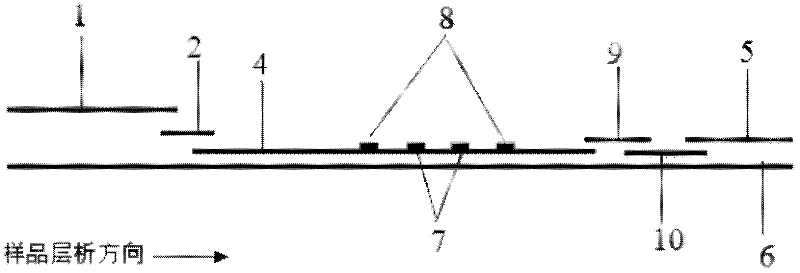
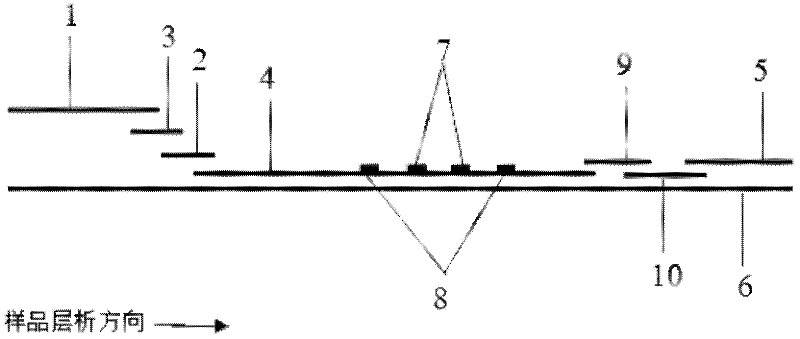
[0088] Example 2: Quantitative detection of CRP by modifying antibody to quantum dots with biotin-avidin system and using indirect pre-wetting immunochromatography
[0089] (1) Modification of quantum dots and antibodies
[0090] First, 1 mL of CRP monoclonal antibody (1 mg / mL) was fully dialyzed with pH 9.0 sodium bicarbonate buffer, and 20-120 μl of N-hydroxysuccinimide biotin ester freshly prepared in dimethyl sulfoxide (DMSO) was added (NHSB, 1mg / mL), react at room temperature in the dark for 4h. Add 1mol / L NH 4 Cl, shaking reaction at room temperature for 10 minutes, then put the solution in a dialysis bag, dialysis and purification overnight, and concentrate with an ultrafiltration centrifuge tube to prepare the required concentration, and the obtained biotinylated CRP monoclonal antibody.
[0091] Streptavidin-modified quantum dots with an emission wavelength of 900nm and biotinylated CRP monoclonal antibody were mixed and reacted at a ratio of 1:3-1:12 for 30-60 minu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Emission wavelength | aaaaa | aaaaa |
| Emission wavelength | aaaaa | aaaaa |
| Emission wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com