Protein chip for lyme disease flagellin antigen immunoserology diagnosis and preparation method and application of protein chip
A protein chip and flagellar antigen technology, applied in the field of protein chips, can solve the problems of easy misdiagnosis and long detection time, and achieve the effects of increased accuracy, high specificity, stability and repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1, gold foil chip surface chemical modification
[0051] Step 1. Cleaning of gold foil chips: by NH 3 :H 2 o 2 :H 2 O=1:1:5 volume ratio mixed to obtain TL1 cleaning solution. Put the gold foil chip in a stainless steel cleaning box filled with TL1 cleaning solution, bathe in 82°C water for 6 minutes, wash twice, take it out, rinse it with ultrapure water for 2 minutes, twice, and then wash it with absolute ethanol for 2 minutes, twice , and then blow dry with nitrogen, and place it in a clean and airtight chip box for use.
[0052] Step 2. Chemically modify the surface of the cleaned gold foil chip to obtain a solid phase carrier
[0053] Immerse the cleaned gold foil chip in modification solution 1, and incubate with shaking at room temperature for 12 hours in the dark. After taking it out, wash it with absolute ethanol solution for 3 times, each time for 2 minutes, and then dry it with nitrogen gas; In the modification solution 2, shake and incubate ...
Embodiment 2
[0055] Embodiment 2 quality control experiment
[0056] Preparation of incubation solution 1: Dissolve Borrelia burgdorferi recombinant flagellar antigen in PBST-BSA solution, and configure the protein concentration gradient to be 200 μg / mL, 100 μg / mL, 50 μg / mL, 25 μg / mL, 12.5 μg / mL, 6.25 μg / mL mL, 3.12μg / mL, 1.65μg / mL, 0.78μg / mL, 0.39μg / mL, 0.19μg / mL flagellar antigen solution.
[0057] Prepare incubation solution 2: Dissolve rabbit anti-Borrelia burgdorferi flagellar antigen IgG antibody in PBST-BSA solution, and configure the antibody concentration gradient to be 200 μg / mL, 100 μg / mL, 50 μg / mL, 25 μg / mL, 12.5 μg / mL, 6.25μg / mL, 3.12μg / mL, 1.65μg / mL, 0.78μg / mL, 0.39μg / mL, 0.19μg / mL solution.
[0058] Prepare incubation solution 3: Dissolve Cy3-labeled goat anti-rabbit IgG antibody in PBST-BSA solution, and configure the fluorescent secondary antibody concentration gradient as 5000μg / mL, 2500μg / mL, 1250μg / mL, 625μg / mL, 312.5μg / mL, 156.2 Solutions of μg / mL, 78.1μg / mL, 39.1μg / ...
Embodiment 3
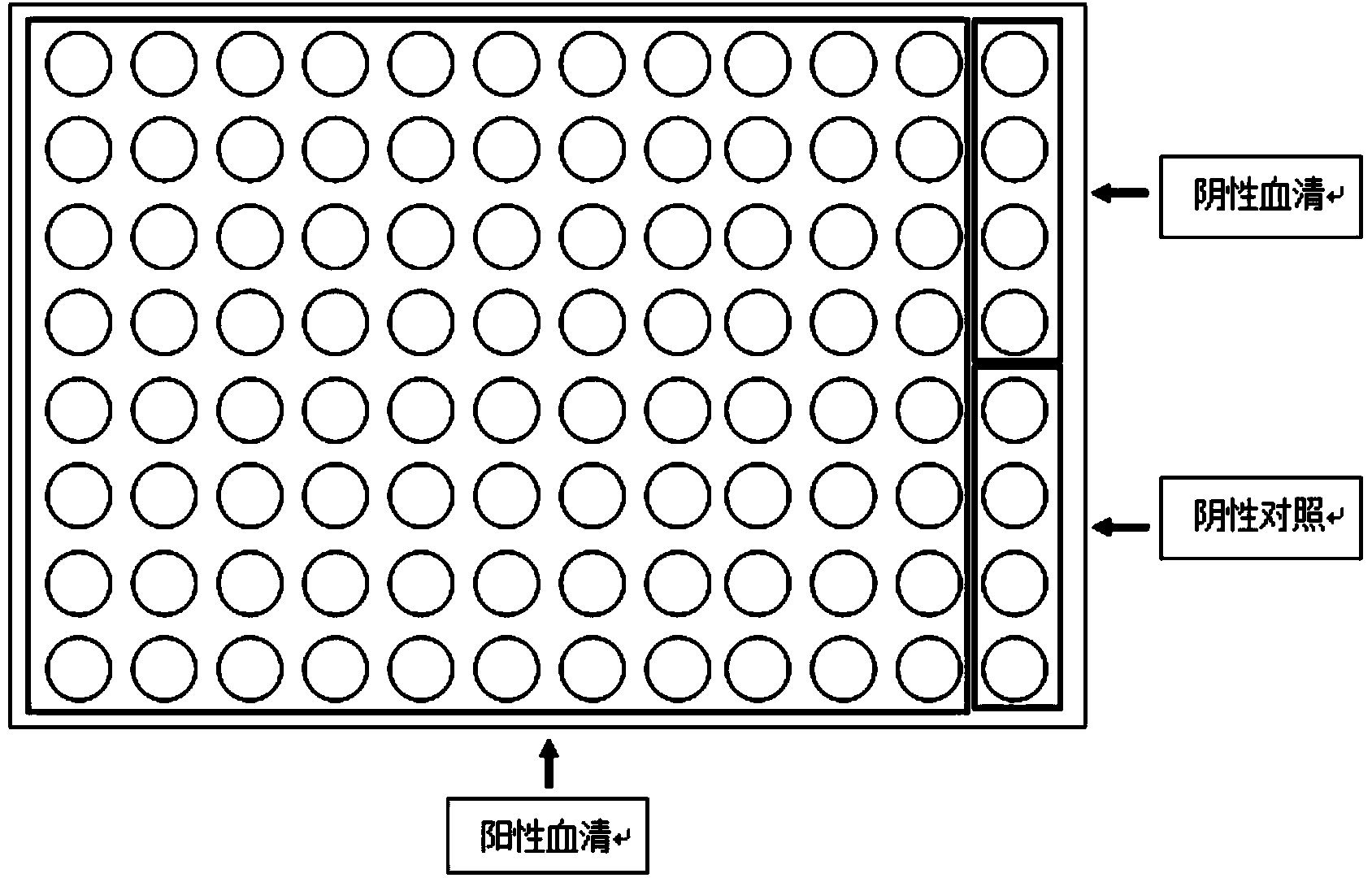
[0077] Example 3 Detection of Serum Samples from Lyme Disease Patients
[0078] The sera of 88 patients with Lyme disease who were clinically diagnosed with Borrelia burgdorferi infection were spotted on 88 wells of the chip containing the probe of recombinant flagellar antigen of Borrelia burgdorferi at an incubation concentration of 50 μg / mL, and the other 4 wells The sample solution of the wells was the serum of healthy people who were not clinically confirmed to be infected with Borrelia burgdorferi, and the sample solution of the remaining 4 wells was PBST-BSA solution, incubated at room temperature for 1 hour, and washed 3 times with PBST after taking it out, 2 minutes each time , blow dry with nitrogen. Do the same on another identical chip. Then dissolve the Cy3-labeled donkey anti-human IgG antibody diluted 1:2500 and the Cy3-labeled goat anti-human IgM antibody diluted 1:2500 in PBST-BSA solution, and apply the samples on two chips incubated with antibodies, and tak...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com