Method and kit for quantitative combined detection of PA (Prealbumin) and CRP (C-reactive Protein), as well as preparation method and application of kit
A combined detection and kit technology, applied in the field of medical testing, can solve the problems of single evaluation index and low clinical acceptance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of the kit for quantitative joint detection of PA and CRP in samples
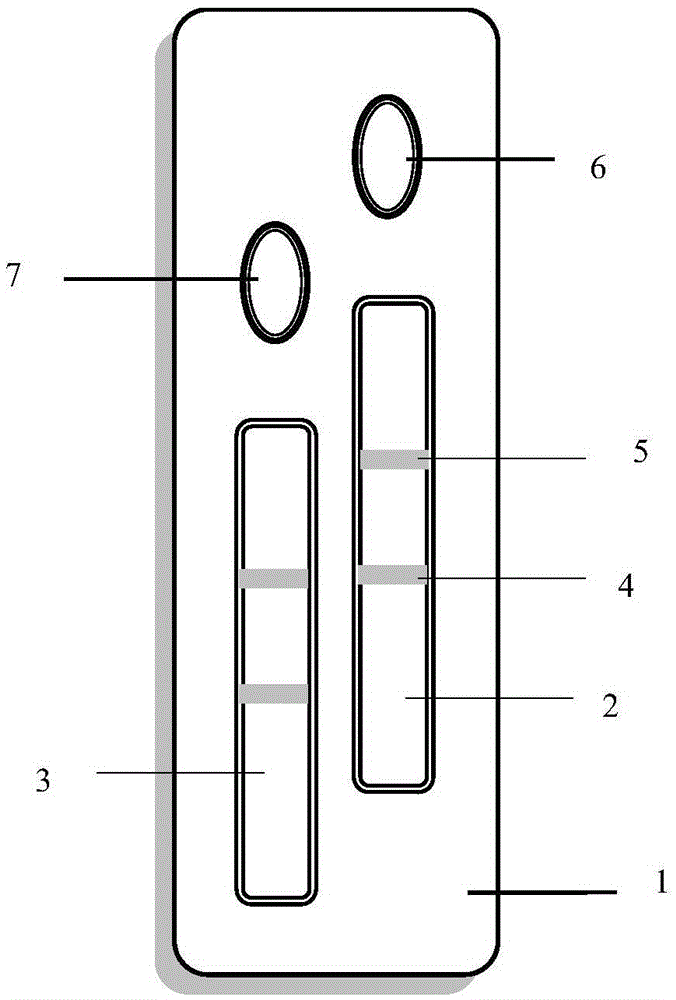
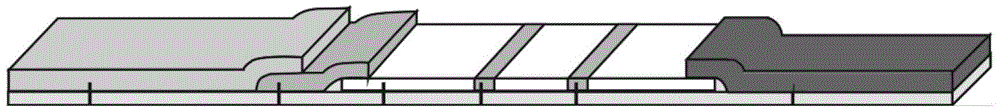
[0062] In this example, the bottom plate material is PVC plastic sheet, which is purchased from Yuyao Fucheng Plastic Chemical Co., Ltd.; the material of the sample pad is glass fiber film, which is purchased from Shanghai Jinbiao Biotechnology Co., Ltd.; the material of the bonding pad is glass The fiber membrane was purchased from Shanghai Jinbiao Biotechnology Co., Ltd. Anti-PA antibody I and anti-PA antibody II have different antigen-binding sites, and anti-CRP antibody I and anti-CRP antibody II have different antigen-binding sites.
[0063] (1) Preparation of nitrocellulose membrane
[0064] Use PB buffer (with 3% sucrose) at pH 7.2 to 7.4 to adjust the concentration of anti-PA antibody II to 0.5 mg / mL, and the concentration of donkey anti-mouse antibody to 1.0 mg / mL, using a Bio-Dot XYZ3050 three-dimensional spraying platform Coat the anti-PA antibody II and donkey anti...
Embodiment 2
[0071] Quantitative detection of PA and CRP in the sample of embodiment 2
[0072] Use the test strip prepared in embodiment 1 to detect PA and CRP in the sample, and its steps include:
[0073] (1) Add solutions containing PA standards with different known concentrations (specifically 0.0, 2.5, 5.0, 12.5, 25.0, 37.5, 75.0, 100, 200 μg / L) to the sample well of the sample pad 1, in the sample Add solutions containing CRP standards with different known concentrations (specifically 0.0, 1.0, 2.5, 5.0, 7.5, 10.0, 50.0, 100.0, 200.0, 400.0 μg / L) to the sample well of pad II, and react for 15 minutes. Place the test strip under a 470nm excitation light source, measure the fluorescence intensity of the strips on the detection line I and quality control line I, and the fluorescence intensity of the strips on the detection line II and quality control line II respectively, according to the detection line I and the quality control line respectively. The ratio of the fluorescence intensi...
Embodiment 3
[0075] Quantitative detection of prealbumin and C-reactive protein in the sample of Example 3
[0076] Take the test strip prepared in Example 1, open the aluminum foil bag and take out the test strip, place it flat on the table, take 2 μL of blood sample in the CRP reagent bottle filled with 2mL of 0.01M pH 7.4 phosphate buffer solution, mix thoroughly and separate Take 100μL and add it to the CRP reagent bottle filled with 0.9mL 0.01M pH 7.4 phosphate buffer solution, take 100uL solution in the reagent bottle and add it to the sample hole of the test strip corresponding to the detection of PA and CRP. After 15 minutes of reaction, put the test paper Place the strips under the excitation light source, measure the fluorescence intensity of the strips on the detection line I and quality control line I, and the fluorescence intensity of the strips on the detection line II and quality control line II respectively, and compare the detection line I and the quality control line I Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com