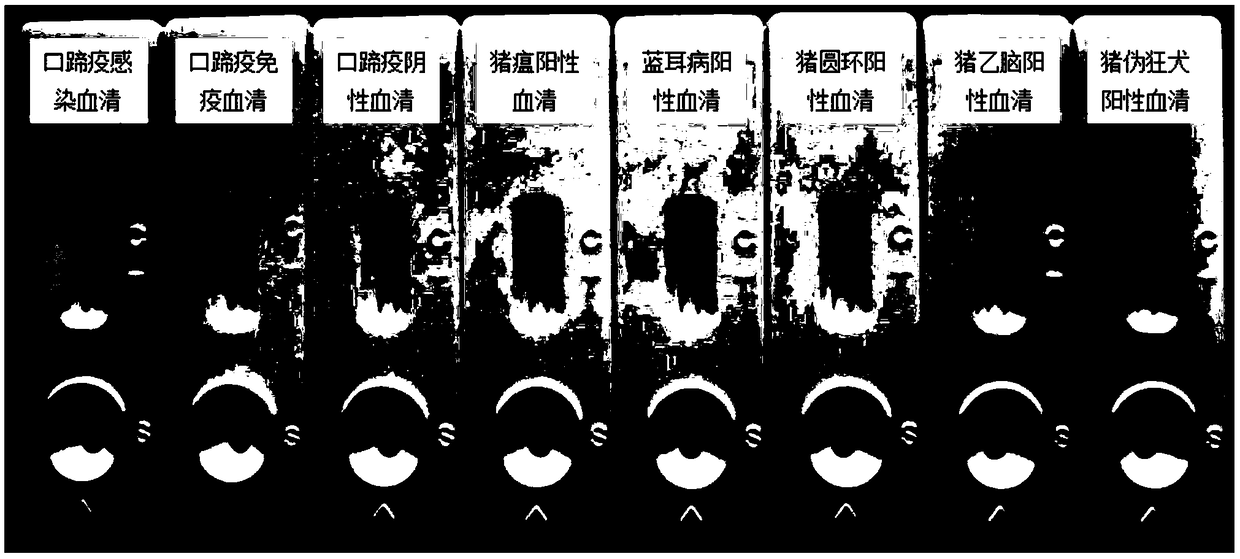
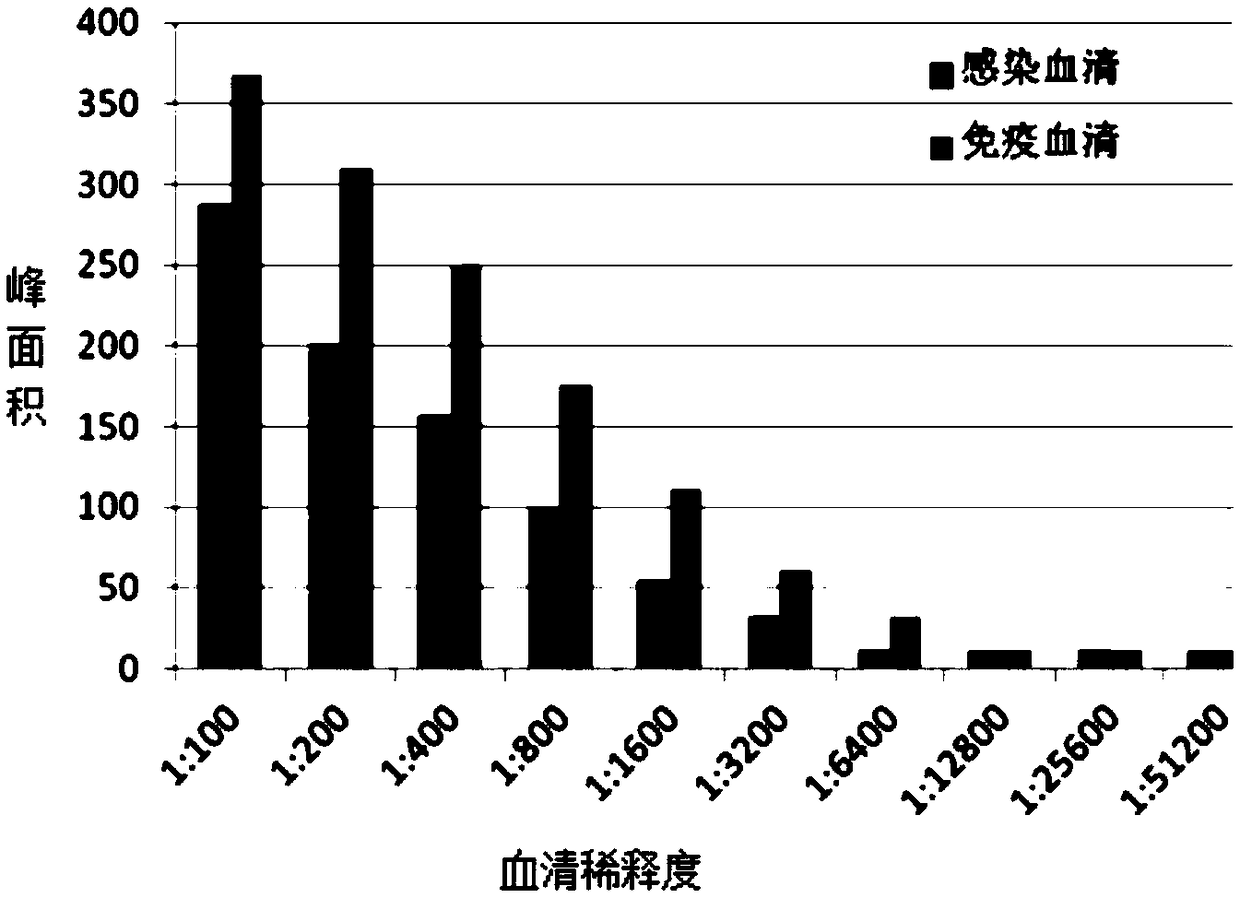
Bigeminal test strip for foot-and-mouth disease virus immune antibody evaluation and infection-immunity differential diagnosis
A foot-and-mouth disease virus and differential diagnosis technology, which is applied in the field of foot-and-mouth disease virus immune antibody evaluation and infection and immune differential diagnosis dual-combination test strips, can solve the problem of affecting the detection accuracy, sensitivity and shelf life of test strip products, and the specific inactivation of viruses in test results Problems such as incomplete virus inactivation and difficulty in ensuring the natural spatial structure achieve the effects of facilitating antigen-antibody reaction, protection stability, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of foot-and-mouth disease virus immune antibody evaluation and infection and immune differential diagnosis dual test strips: First, according to the B-cell epitope on the foot-and-mouth disease virus VP1 and the B-cell epitope on the non-structural protein of the foot-and-mouth disease virus, artificially synthesize the foot-and-mouth disease virus structural protein VP1 on the table Epitope polypeptides and 3 epitope polypeptides on foot-and-mouth disease virus non-structural proteins 2B, 2C, and 3B are coupled to carrier proteins, respectively, for the preparation of detection line 1 (T1) and detection line 2 (T2) on cellulose membrane mats, and prepared simultaneously The secondary antibody IgG against the animal species to be tested is used to prepare the control line (C) on the cellulose membrane pad; the gold standard pad treatment solution and the gold standard pad pretreatment are prepared for spraying the gold standard protein; finally, the test strip...
Embodiment 2
[0059] Foot-and-mouth disease virus immune antibody evaluation and the preparation method of infection and immune differential diagnosis dual test strips are as in Example 1, and the 142nd to 158th amino acid sequence NNVRGDLQVLAQKAERA on the detection line 1 is sprayed on the foot-and-mouth disease virus O / GX / 09-7 strain VP1 (SEQ ID NO.1), adding a cysteine at its -NH4 end, coupling the artificial antigen prepared by carrier protein BSA or OVA; spraying the artificial antigen of non-structural protein of foot-and-mouth disease virus on the detection line 2, and the artificial antigen is selected at the same time The 1096th to 1106th amino acid (amino acid sequence: RTPEDLERAEK, SEQID NO.5) polypeptide on the nonstructural protein 2B of foot-and-mouth disease virus, and the 1414th to 1425th amino acid (amino acid sequence: HEKVSSHPIFKQ, SEQID NO.6) on the nonstructural protein 2C Polypeptide, amino acid 1602-1613 on non-structural protein 3B (amino acid sequence: GPYAGPMERQKP...
Embodiment 3
[0061] Foot-and-mouth disease virus immune antibody evaluation and the preparation method of infection and immune differential diagnosis dual test strips are as in Example 2, the difference is that the artificial antigen polypeptide on the detection line 1 is on the foot-and-mouth disease virus O / HN / CHA / 93 strain VP1 The 142nd-158th amino acid sequence SNVRGDLQVLAQKAERA (SEQ ID NO.2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com