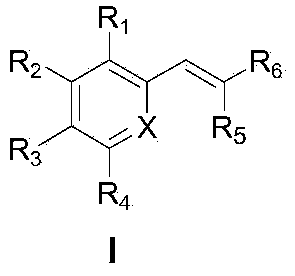
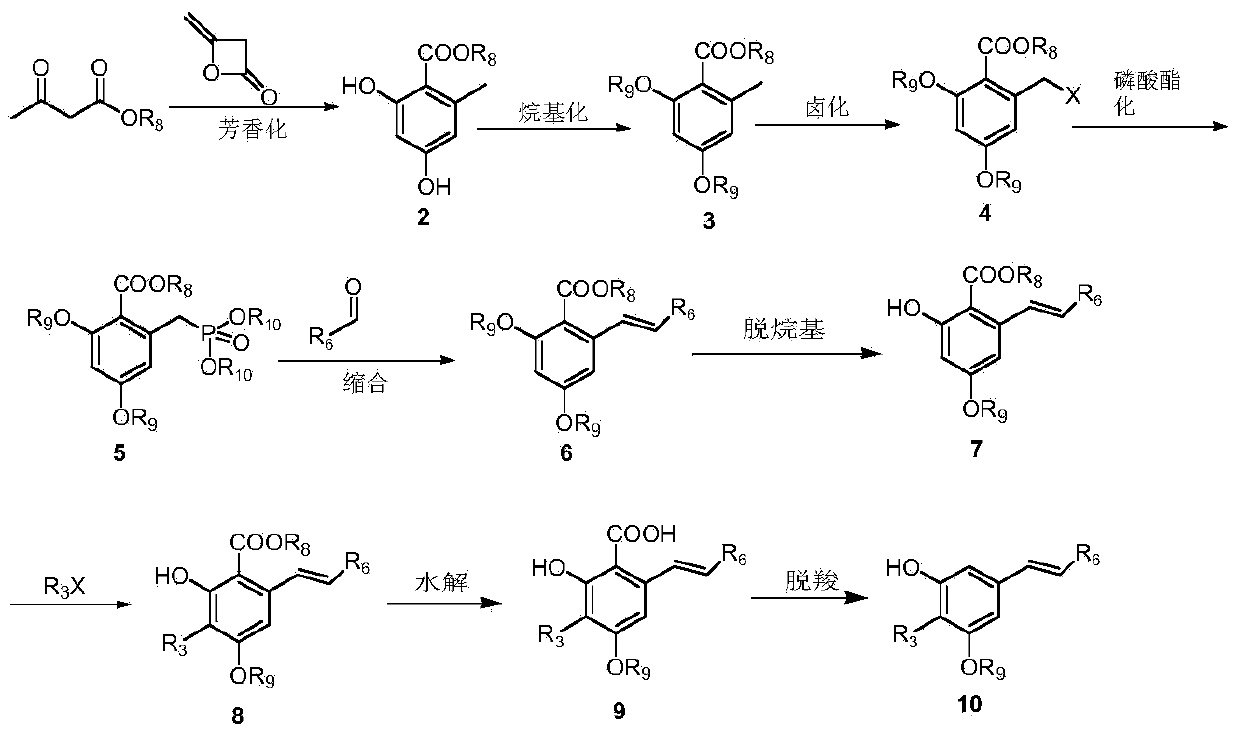
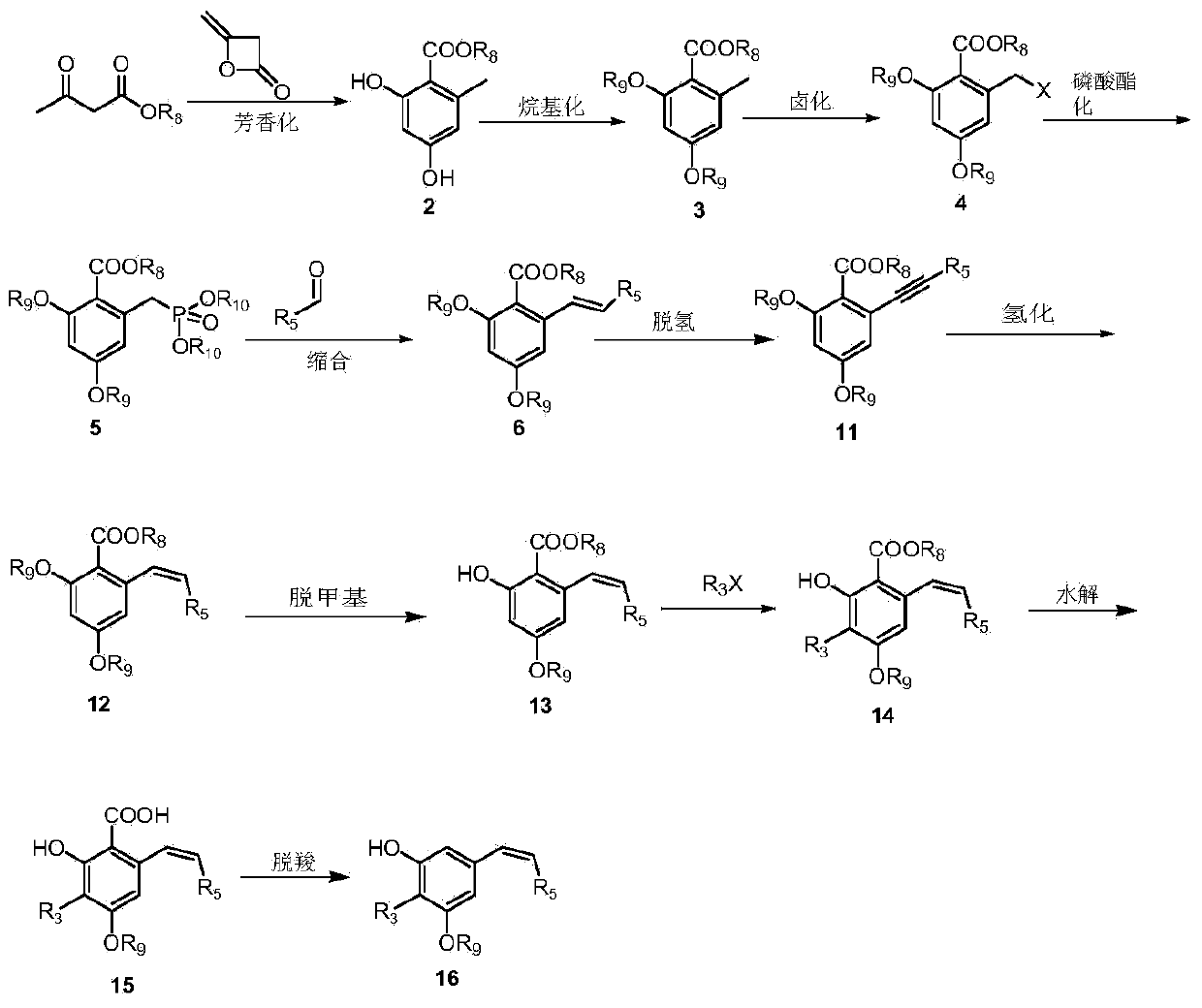
Diarylethene structure similar compounds as well as preparation method and application thereof
A compound and vinyl technology, applied in the field of chemical total synthesis of natural product kalinin analogues, can solve the problems of in-depth research on the pharmacological effects of natural product kalinin, the limited number of analogues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Preparation of methyl 2,4-dihydroxy-6-methylbenzoate (2)
[0103] Methyl acetoacetate (50g, 0.43mol) was dissolved in 300ml of ether, NaH (15.50g0.45mol, 70%) was added at room temperature, after the addition, the ether solution of diketene (37g, 0.45mol) was added dropwise at this temperature After the addition, react at room temperature for 3-4 hours, at which time the reaction system becomes yellow turbid liquid. The reaction was terminated, the reaction solution was added to 500 ml of ice-water mixture, the ether layer was separated, the water layer was extracted twice with 50 ml of ether, the ether layers were combined, washed with saturated brine, and dried overnight with anhydrous magnesium sulfate. Filtration, rotary evaporation to remove the ether, the residue was passed through a silica gel column with petroleum ether: ethyl acetate = 8:1 to obtain 35 g (45%) of white solid as the target product. 1 H-NMR(400M,DMSO-d 6 )δ(ppm): 10.66(s,1H),9.95(s,1H), 6.16(d,J=2.4...
Embodiment 2
[0105] Preparation of 2,4-Dimethoxy-6-methylbenzoic acid methyl ester (3)
[0106] Compound 2 (12g, 0.066mol) was dissolved in 50ml of acetone, potassium carbonate (27.3g, 0.198mol), methyl iodide (28g, 0.198mol) were added, and the reaction was heated to reflux for 3h. The reaction was stopped. The reaction solution was added to 100ml of water and acetic acid Extract with ethyl acetate (350 ml), combine the organic layers, and wash the organic layers with 10% sodium hydroxide solution, 10% hydrochloric acid, and saturated brine in sequence. The organic layer was dried with anhydrous magnesium sulfate. After filtration, the solvent was removed by rotary evaporation to obtain a colorless oil, which was recrystallized with petroleum ether / ethyl acetate to obtain 13.5 g (97%) of colorless crystals. 1 H-NMR(400M,CDCl 3 )δ(ppm): 6.31(s, 2H), 3.88(s, 3H), 3.80(s, 6H), 2.28(s, 3H)
Embodiment 3
[0108] Methyl 2-bromomethyl-4,6-dimethoxybenzoate (4)
[0109] Compound 3 (10g, 0.0476mol) was dissolved in 50ml of carbon tetrachloride, protected by nitrogen, heated to reflux, and a mixture of NBS (8.5g, 0.0476mol) + BPO (0.11g, 0.476mmol) was added in batches. After the addition, reflux Reaction for 1h. The reaction was stopped, cooled, filtered, and the filtrate was spin-dried to obtain a pale yellow solid, which was recrystallized from absolute ethanol to obtain 11.3 g (82%) of a white solid. 1 H-NMR(400M,CDCl 3 )δ(ppm): 6.74(s, 1H), 6.47(s, 1H), 4.66(s, 2H), 3.96(s, 3H), 3.93(s, 3H), 3.85(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com