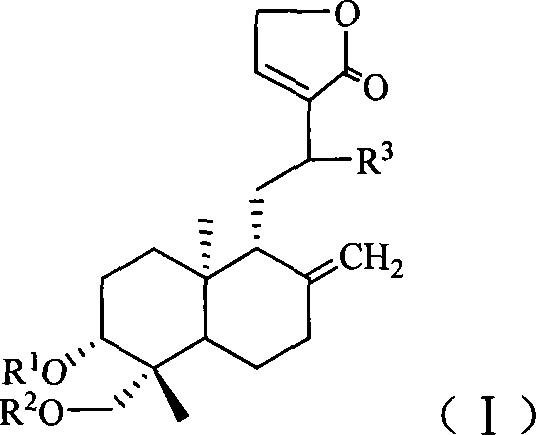
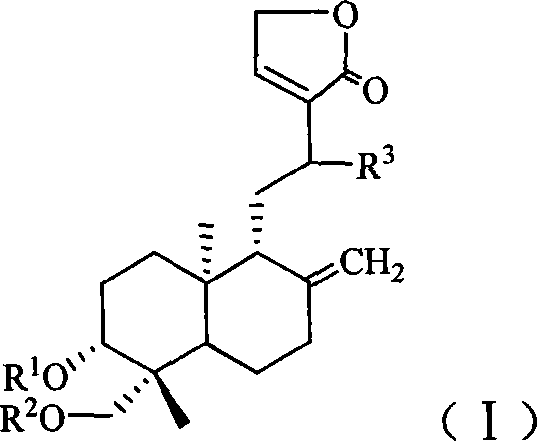
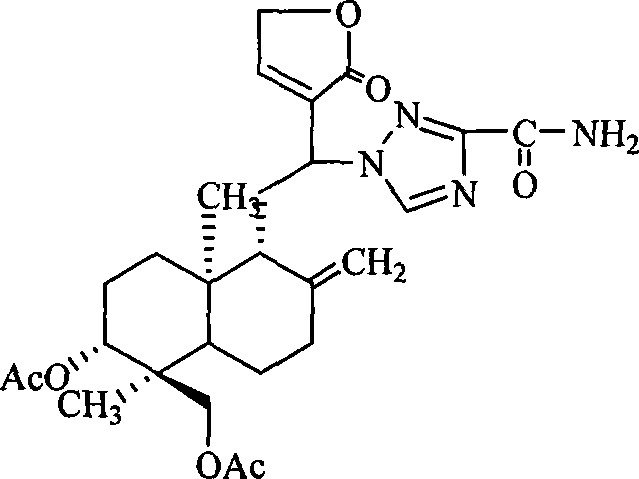
Antiviral compound and preparation method thereof
A compound and pharmaceutical technology, applied in the field of medicine, can solve problems such as the lack of effective therapeutic drugs, the inability to directly kill viruses, and the increase in demand for therapeutic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] The preparation of embodiment 1 compound of the present invention
[0122] 1. Preparation of 3,19-diacetyl-12-(N-3-formylamino-1H-1,2,4-triazole)-14-deoxy-andrographolide (compound 1 for short)
[0123] Put 3.5g (10mmol) of andrographolide into the reaction flask, then add 50ml of acetic anhydride, reflux for 10min, recover the acetic anhydride after the reaction is completed, pour the residue into 200ml of ice water, extract with 50ml of chloroform × 2, combine the organic layers, Washed with water, dried, and the solvent was recovered under reduced pressure to obtain a crude product of 3,14,19-triacetyl-andrographolide, which was recrystallized from absolute ethanol to obtain 3.4 g of a refined product, yield: 71%.
[0124] Dissolve 1.1g (10mmol) 3-formylamino-1H-1,2,4-triazole in 20ml chloroform, add 2.7g (25mmol) / 20ml chloroform containing trimethylchlorosilane dropwise, and stir at room temperature for reaction 3 Hour. The reaction solution was transferred into...
Embodiment 2
[0149] The preparation of embodiment 2 compound tablet of the present invention
[0150] Prescription 1:
[0151] Any one of compounds 1-7 or its derivatives 100g (based on compound)
[0152] Microcrystalline Cellulose 50g
[0153] 60g pregelatinized starch
[0154] Magnesium Stearate 1.5g
[0155] Carboxymethyl Starch Sodium 5g
[0156]
[0157] A total of 1000 tablets were prepared
[0158] Prescription 2:
[0159] Any one of compounds 1-7 or its derivatives 200g (based on compound)
[0160] Microcrystalline Cellulose 100g
[0161] 120g pregelatinized starch
[0163] Carboxymethyl Starch Sodium 10g
[0164]
[0165] A total of 1000 tablets were prepared
[0166] 2. Preparation process: pass the raw materials and auxiliary materials through 100 mesh sieve respectively, and set aside; weigh the raw materials and aux...
Embodiment 3
[0167] The preparation of embodiment 3 compound capsules of the present invention
[0168] 1. Prescription
[0169] Prescription 1:
[0170] Any one of compounds 1-7 or its derivatives 50g
[0171] 20g pregelatinized starch
[0172] Microcrystalline Cellulose 10g
[0173] 10% PVPK30 ethanol solution appropriate amount
[0174] Magnesium stearate 0.5g
[0175]
[0176] A total of 1000 capsules were prepared
[0177] Prescription 2:
[0178] Any one of compounds 1-7 or its derivatives 75g
[0179] 30g pregelatinized starch
[0180] Microcrystalline Cellulose 15g
[0181] 2% HPMC aqueous solution appropriate amount
[0182] Micronized silica gel 1.5g
[0183] Magnesium stearate 0.75g
[0184]
[0185] A total of 1000 capsules were prepared
[0186] Prescription 3:
[0187] Any one of compounds 1-7 or its derivatives 100g
[0188] 40g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com