HO-1 and HO-1 inducer for inhibiting PRRS virus (PRRSV) infection as novel blocker
A virus infection, HO-1 technology, applied in antiviral agents, medical preparations containing active ingredients, peptide/protein components, etc., can solve the problem of unclear pathogenic mechanism and immune mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The preparation of embodiment 1 CoPP and hemin
[0016] Dissolve CoPP and hemin (both purchased from Sigma, USA) in 0.2 mol / L NaOH, adjust the pH value to 7.4 with 1 mol / L HCL, then dilute with PBS, and filter through a 0.2 μm filter membrane Sterilize, wrap in tinfoil after aliquoting, and store at -80°C for later use.
Embodiment 2
[0017] Example 2 CoPP and hemin treatment of cells infected by PRRSV
[0018] Marc-145 cells and PAM (10 5 cells / ml) were cultured in six-well cell cultures containing DMEM and RPMI-1640 medium (containing penicillin 100 U / ml, streptomycin sulfate 50 μg / ml, gentamicin 50 μg / ml, 10% fetal bovine serum) plate, 37°C, 5% CO 2 Culture in a humidified incubator to a confluence of 70% to 80%, and discard the medium. Insert 0.1MOI of PRRSV into each well, absorb at 4°C for 1 hour (h), incubate at 37°C for 1h, discard unadsorbed virus liquid, and wash once with 1ml PBS / well. Add 2ml of the medium containing CoPP at different concentrations (0μM, 20μM, 40μM, 60μM, 80μM, 100μM) diluted in Example 1 to each well, and place it at 37°C in 5% CO 2 cultured in a humidified incubator until virus infection for 48 hours, and the cell supernatant and cells were collected respectively.
[0019] Marc-145 cells and PAM (10 5 cells / ml) were cultured in six-well cell cultures containing DMEM and ...
Embodiment 3
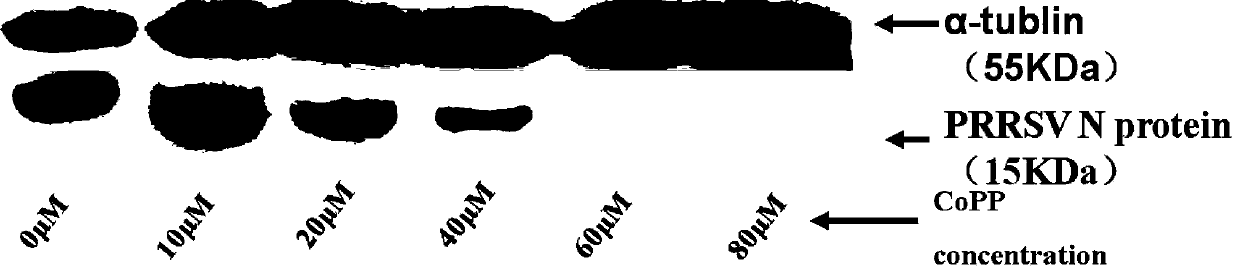
[0020] Example 3 CoPP and hemin inhibit the replication of PRRSV genome and the expression of nucleocapsid protein
[0021] Real-time fluorescent quantitative PCR detection of N gene expression of HO-1 and PRRSV: After the cells treated in Example 2 were trypsinized, they were collected in a 1.5ml centrifuge tube, centrifuged at 500g for 10min at 4°C, and the supernatant was discarded. Add 1ml TRI zol (purchased from Invitrogen) to each tube, and extract RNA according to the instructions. RNA was reverse-transcribed into cDNA using PrimeScript RT reagent Kit Perfect Real Time kit (purchased from TAKARA). Finally, apply the SYBR GREEN kit (purchased from Roche Company in Switzerland) and the above reverse-transcribed cDNA template and the designed upstream and downstream primers of HO-1 and N genes, and perform real-time PCR on the StepOnePlus Real-Time PCR System of Applied Biosystems. Fluorescent quantitative PCR detection. The reaction conditions were as follows: denaturat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com