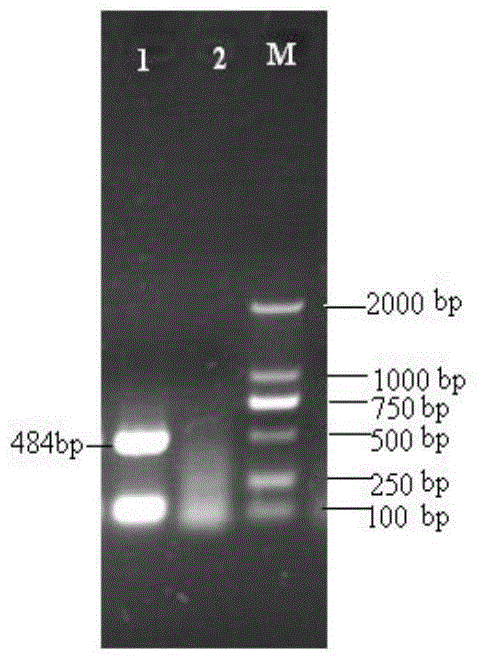
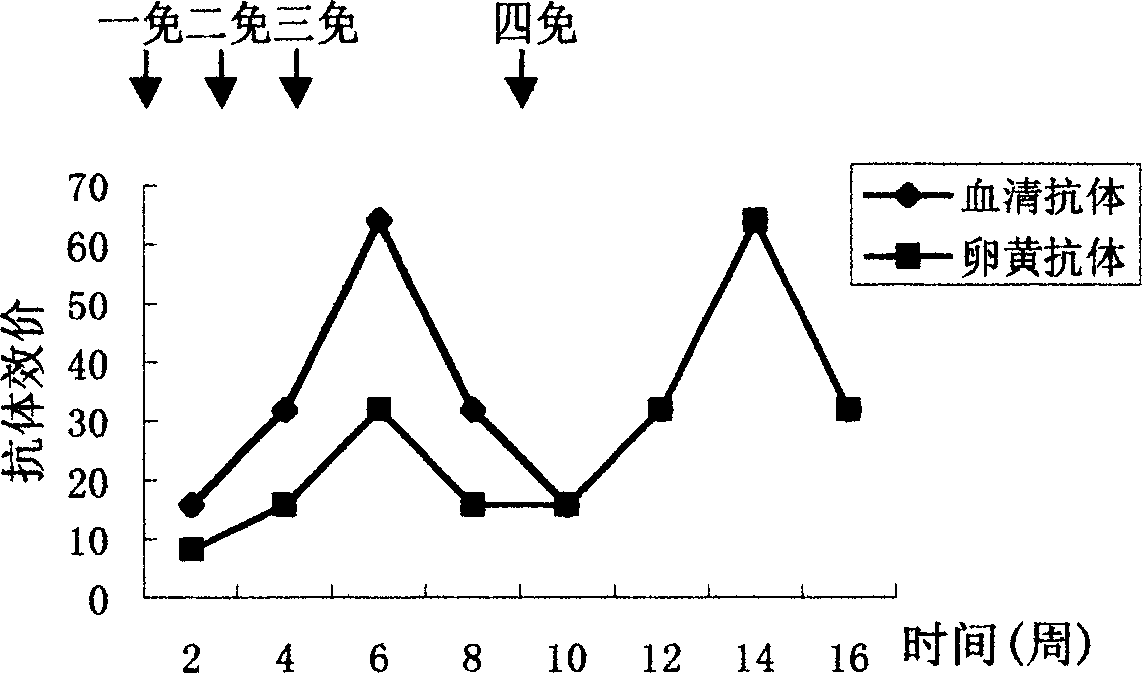
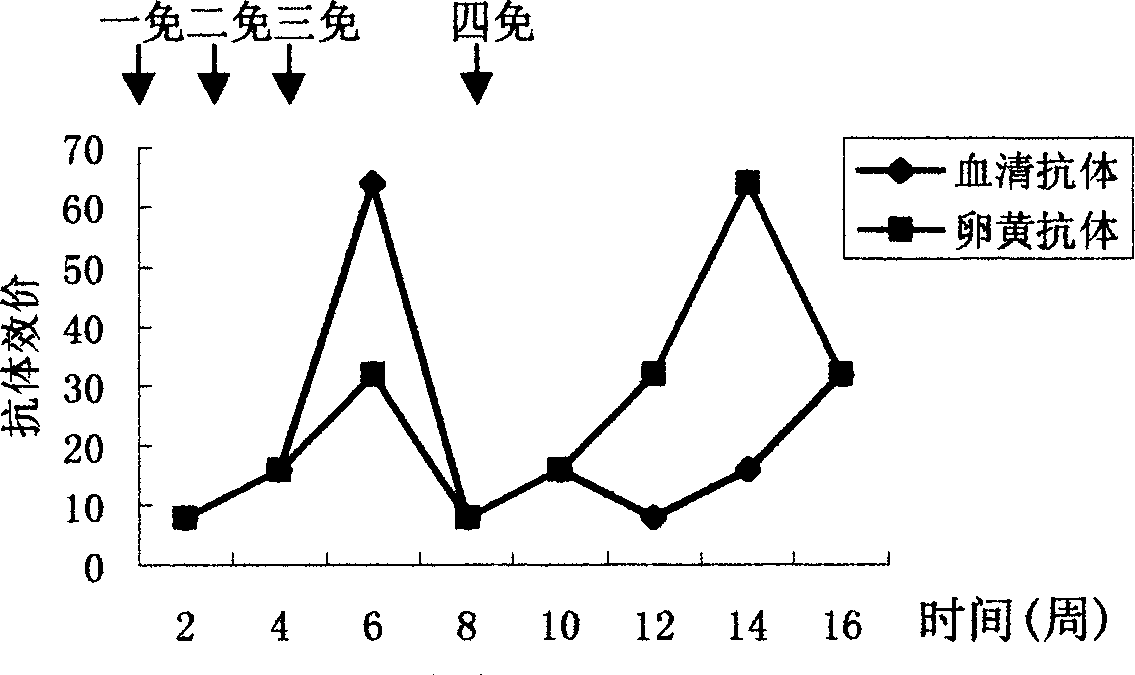
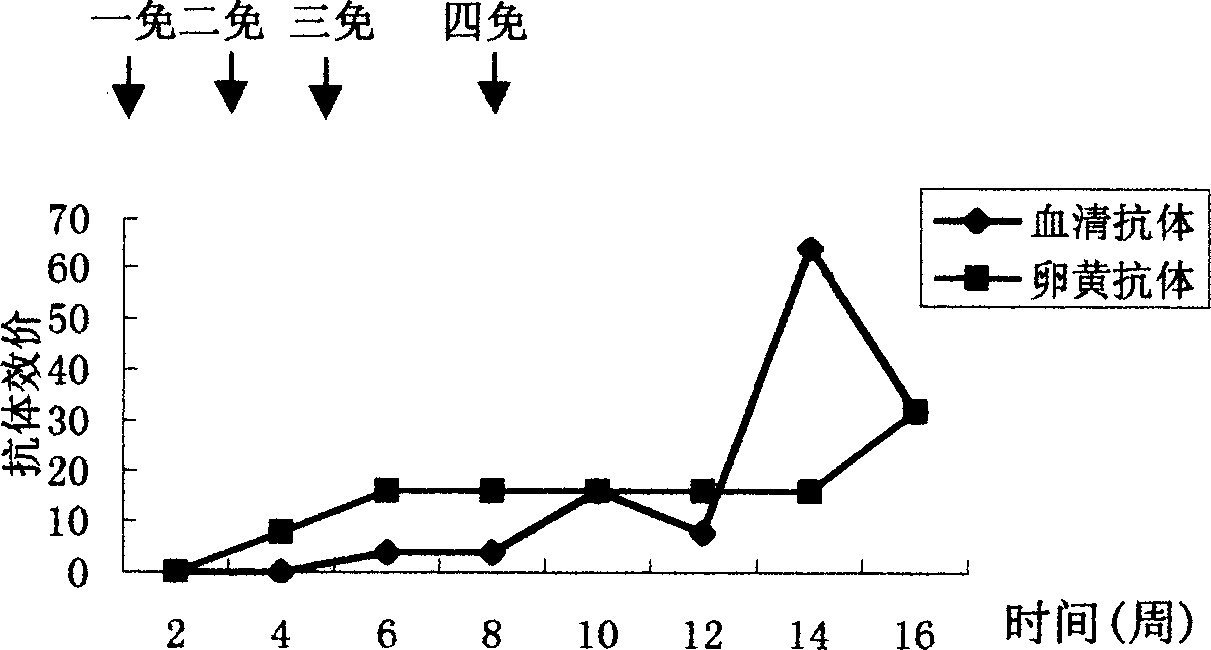
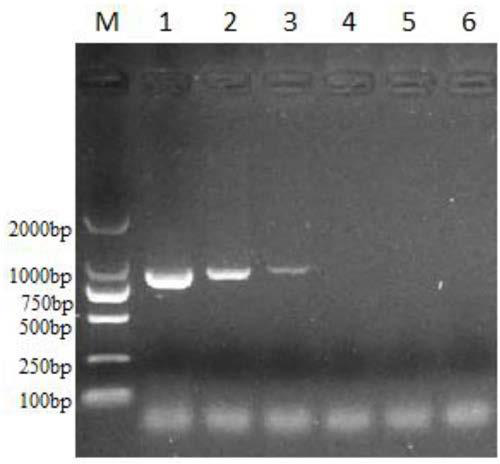
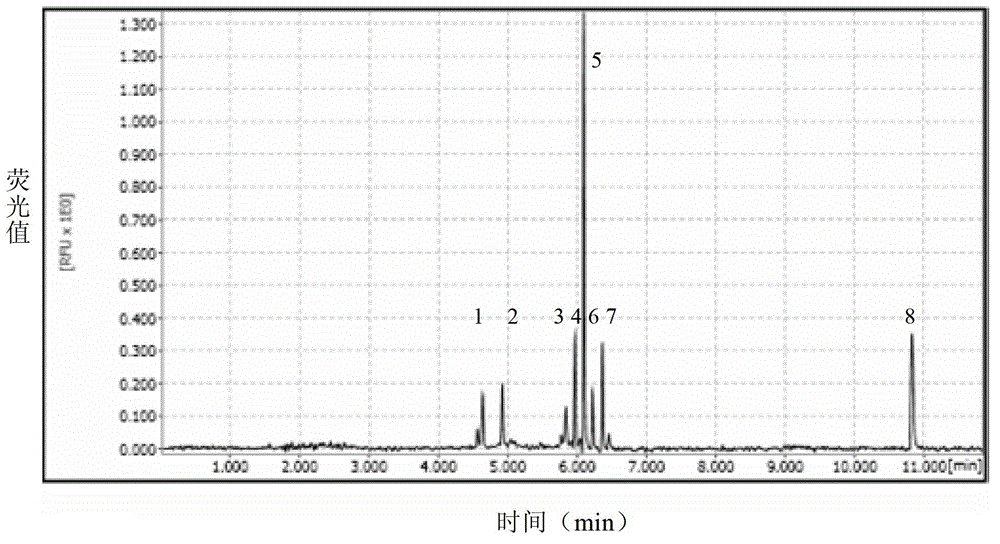
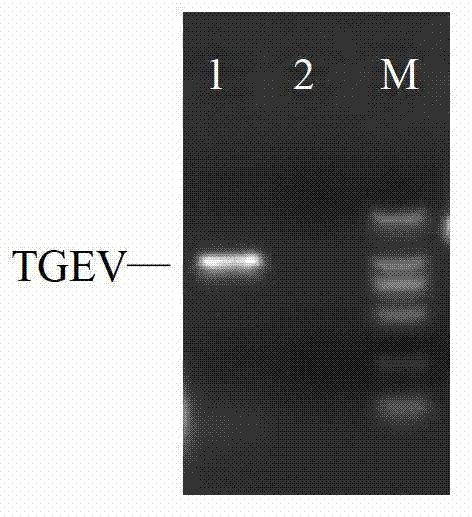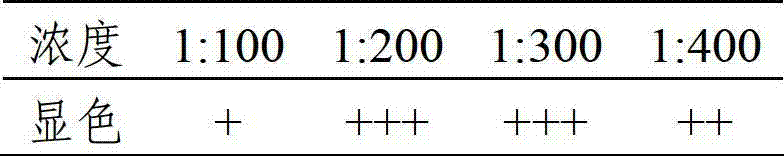Patents
Literature
142 results about "Porcine transmissible gastroenteritis virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
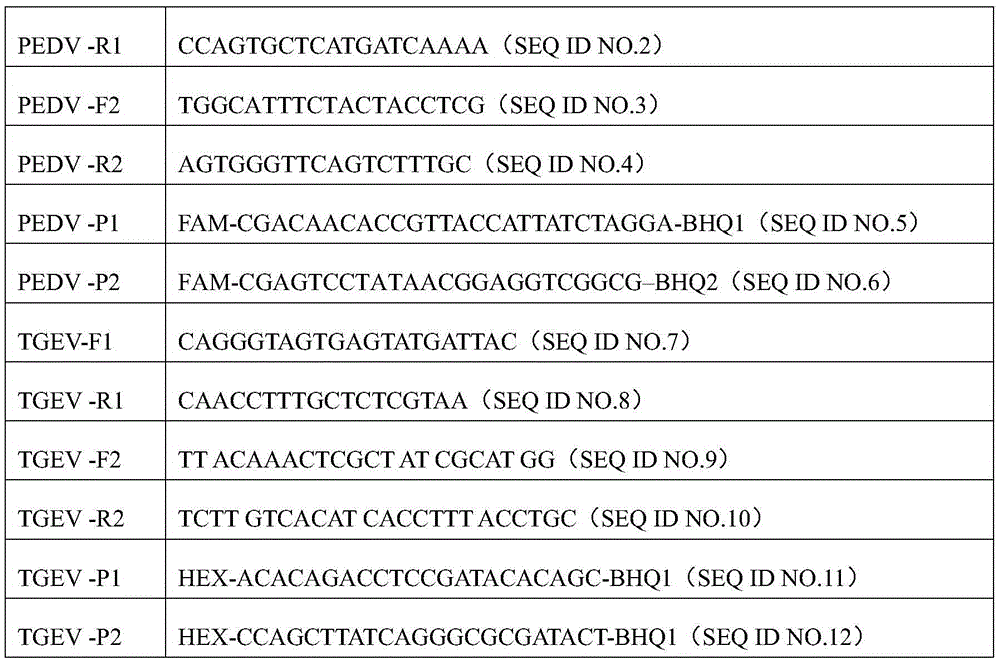
Primers, probes and detection kits for detection of porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus
InactiveCN102277454AShorten the timeSave costsMicrobiological testing/measurementFluorescence/phosphorescenceDuplex pcrDiarrhea
The invention discloses a primer, a probe and a detection kit for detecting porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. The detection primers and probes are SEQ ID NO: 1 to SEQ ID NO: 6 in the sequence table, wherein the sequences SEQ ID NO: 1 and SEQ ID NO: 2 are sense primers and antisense primers for detecting porcine transmissible gastroenteritis virus respectively, and the sequence SEQ ID NO: 3 For detecting the fluorescent probe of porcine transmissible gastroenteritis virus, sequence SEQIDNO: 4 and SEQIDNO: 5 are sense primer and antisense primer for detecting porcine epidemic diarrhea virus respectively, and sequence SEQIDNO: 6 is the detection primer of porcine epidemic diarrhea virus fluorescent probe. The invention also provides detection kits for porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. The primers and probes selected by the present invention have very strong specificity. The total viral RNA extracted from porcine diarrhea does not need to be transcribed into cDNA first. The synthesis of the first strand of cDNA and double PCR are completed in one step, and two viruses can be detected at one time. ,Improve efficiency.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
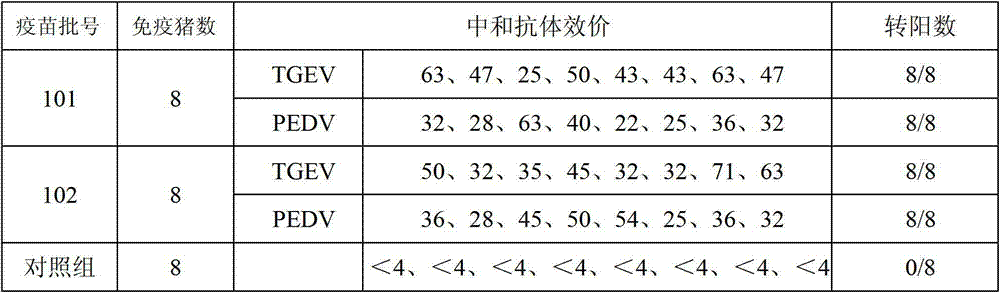
Porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and preparation method thereof
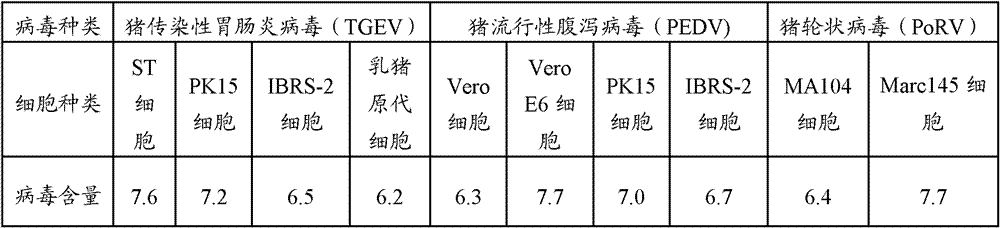
The invention relates to a porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine and a preparation method of the porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine is prepared by performing virus amplification on a swine testicular cell line (ST cells) or an African green monkey kidney cell line (Vero cells) by using a self-attenuated and preserved transmissible gastroenteritis virus SD / L strain and a self-attenuated and preserved porcine epidemic diarrhea virus LW / L strain, and carrying out the steps of harvesting, uniformly mixing, freeze-drying and the like. The porcine transmissible gastroenteritis and epidemic diarrhea combined live vaccine can effectively prevent two diseases namely swine transmissible gastroenteritis and epidemic diarrhea.
Owner:QILU ANIMAL HEALTH PROD
Triple vaccine of pig transmissible gastroenteritis, pig epidemic diarrhea and pig rotavirus
InactiveCN101491673AAvoid pollutionDoes not destroy nutrientsViral antigen ingredientsDigestive systemDiseaseCytopathic effect
The invention provides a method for preparing triple vaccine for preventing porcine transmissible gastroenteritis, porcine epidemic diarrhea and porcine rotavirus. The method comprises the following steps: inoculating a host-cell line with a 90 percent grown monostratum against a porcine transmissible gastroenteritis virus, a porcine epidemic diarrhea virus and a porcine rotavirus respectively, and adding a cell maintenance media into the host-cell lines respectively to be cultured at 37 DEG C; after cytopathic effect reaches over 75 percent, collecting viruses to be stored at 20 DEG C below zero for standby; mixing the viruses according to 10 TCID50 in 1:1:1, and simultaneously adding Freund's complete adjuvant and immunopotentiator into the mixture to inactivate the mixture by formaldehyde at 37 DEG C for 24 hours; and adding an oil adjuvant into the mixture to prepare a vaccine of water-oil-water preparation. The method can be used for preparing the triple vaccine for preventing the porcine transmissible gastroenteritis, the porcine epidemic diarrhea and the porcine rotavirus so as to solve the problem that the diseases do not have an effective medicine to treat currently.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD +1
Triple live vaccine for swine transmissible gastroenteritis virus, swine epidemic diarrhea virus and swine rotavirus
ActiveCN102949718AReduce immune efficiencyReduced immune potencyViral antigen ingredientsAntiviralsEpidemic diarrheaRotavirus RNA
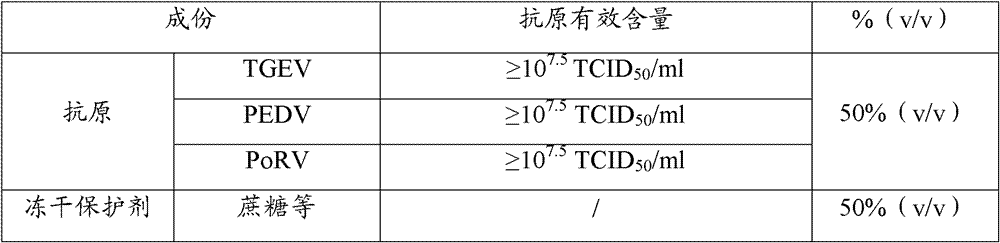
The invention provides a triple live vaccine for a swine transmissible gastroenteritis virus, a swine epidemic diarrhea virus and a swine rotavirus and a preparation method thereof. The content of the three viruses is not less than 107.5 TCID50 (Tissue Culture Infectious Dose 50) / mL, and the volume ratio is 1:1:1. The triple live vaccine provided by the invention solves the problem that a multiple vaccine for effectively preventing and treating such three diseases as swine transmissible gastroenteritis, swine epidemic diarrhea and the swine rotavirus is not available on the current market, and especially realizes the prevention and control on the swine rotavirus. Compared with the existing method of inoculating with three simplex vaccines to prevent such three transmissible diseases, the triple live vaccine provided by the invention is economical to use, simplifies the immunization procedure and lowers the epidemic prevention cost, thereby providing a new simple and convenient immunization way for farms in China.
Owner:PU LIKE BIO ENG
Anti-swine transmissible gastroenteritis virus and porcine epidemic diarrhea virus egg-yolk antibody and preparation method thereof
ActiveCN104788561ANo growthGood physical propertiesEgg immunoglobulinsImmunoglobulins against virusesSwine Transmissible GastroenteritisArtificial infection
The invention discloses an anti-swine transmissible gastroenteritis virus and porcine epidemic diarrhea virus egg-yolk antibody. Swine transmissible gastroenteritis virus and porcine epidemic diarrhea virus combined inactivated vaccine is used as an immunogen to immune laying hens, and an egg-yolk antibody is purified from egg yolk, wherein the swine transmissible gastroenteritis virus is swine transmissible gastroenteritis virus HB08 with the collection number being CGMCC No.7807; and the porcine epidemic diarrhea virus is porcine epidemic diarrhea virus ZJ08 with the collection number being CGMCC No.7806. The prepared egg-yolk antibody has good and safe traits. Artificial infection cure rate of the prepared egg-yolk antibody reaches 100% and is obviously higher than cure rate of an egg-yolk antibody prepared from classical IBDV. Clinical case cure rate of the prepared egg-yolk antibody reaches 93.0%. In clinical preventive tests, incidence of diarrhea can be reduced by 17-24% for tested pigs.
Owner:兆丰华生物科技(南京)有限公司 +3
Yolk antibody and antigen of pig's infective enterogastritis virus and its preparing process
InactiveCN1382736AGood treatment effectNo toxic side effectsEgg immunoglobulinsVirus peptidesYolkAntigen
A yolk antibody for pig's infective enterogastritis virus is prepared through reproducing the said virus on pig's testis cell, applying it as immunogen to the layer, collecting yolk and purifying. When it is applied to pig, the comparison between experimental and reference groups shows that it has high effect on preventing and cuting pig's infective enterogastritis.
Owner:INST OF ZOOTCHNICS & VERTERINARY SCI BEIJING ACAD OF AGRI & FORESTRY SCI
Porcine transmissible gastroenteritis virus (PTGEV) and porcine epidemic diarrhea virus (PEDV) dual yolk antibody and preparation method thereof
ActiveCN101948536AEasy to produceReduce manufacturing costEgg immunoglobulinsDigestive systemYolkFreeze-drying
Owner:PU LIKE BIO ENG
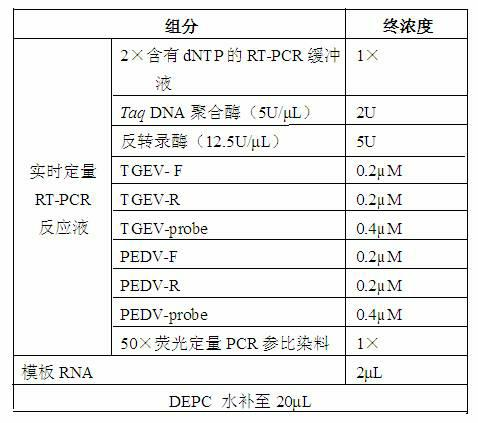
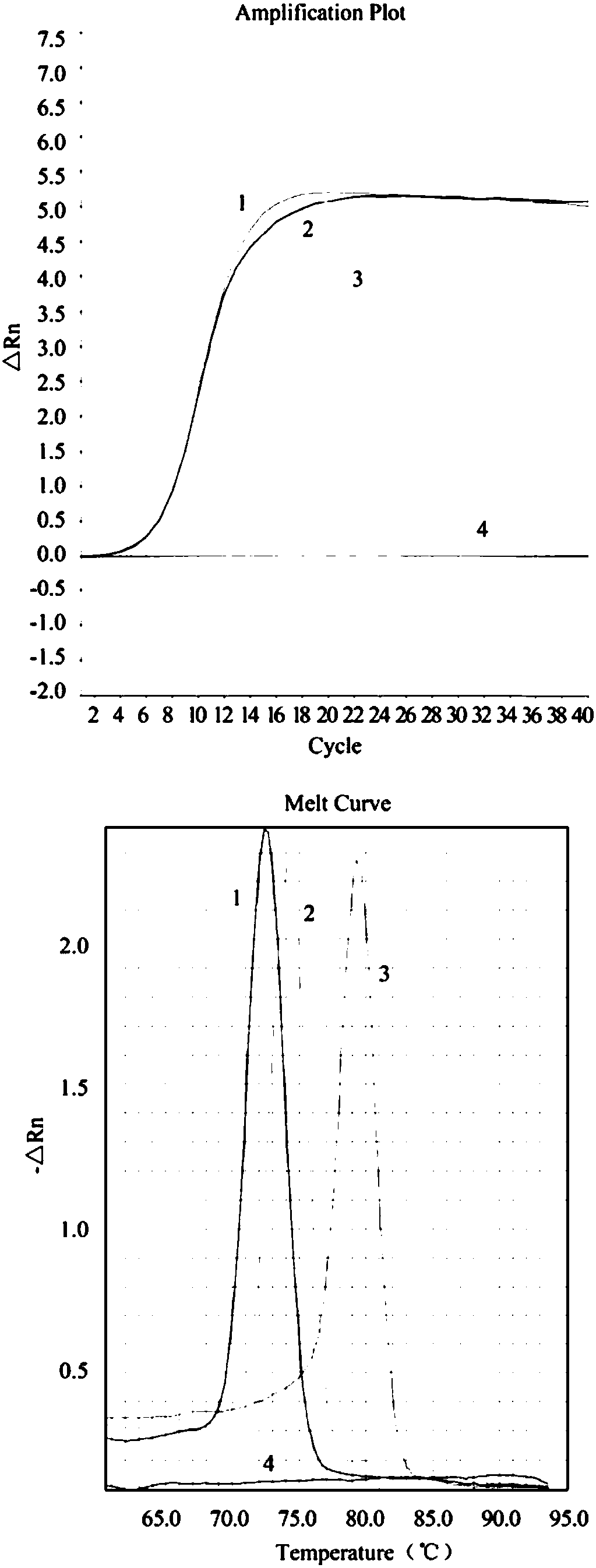
Fluorescence quantitative PCR (Polymerase Chain Reaction) detection method for porcine transmissible gastroenteritis virus gene S and primer thereof
InactiveCN102154516ALow costReduce false positivesMicrobiological testing/measurementMicroorganism based processesCompetent cellFluorescence
The invention discloses a fluorescence quantitative PCR (Polymerase Chain Reaction) detection method for a porcine transmissible gastroenteritis virus gene S and a primer thereof in the technical field of biotechnology. The method comprises the following steps of: cloning a PCR amplification target segment identified as a positive PCR product to a vector pMD18-T, transforming to a competent cell DH5alpha, selecting positive clone by screening blue and white spots and identifying sequencing; extracting a positive recombinant plasmid, quantifying by using an ultraviolet spectrophotometer, diluting a standard product series by 10 times of gradient until the final concentration is 1.0*10<3>-1.0*10<11> copies / mL, undergoing a fluorescence quantitative PCR by taking the standard product series as a template, and establishing a fluorescence quantitative PCR standard curve; and extracting virus RNA (Ribonucleic Acid) of a clinical excrement sample, undergoing a fluorescence quantitative PCR, and calculating the content of viruses in the sample according to a result and the standard curve, wherein the sequences of the primer are sequence 1 and sequence 2. The method and the primer have theadvantages that: a fluorescent probe does not need to be designed additionally, the cost is lowered, operation is easy and convenient, and detection can be completed within 2 hours. The detection method and the primer are suitable for any fluorescence quantitative PCR instrument, and can be applied to the detection of large-scale and high-flux samples.
Owner:SHANGHAI JIAO TONG UNIV
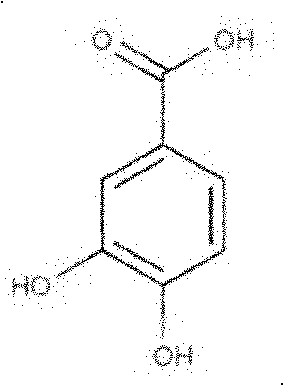
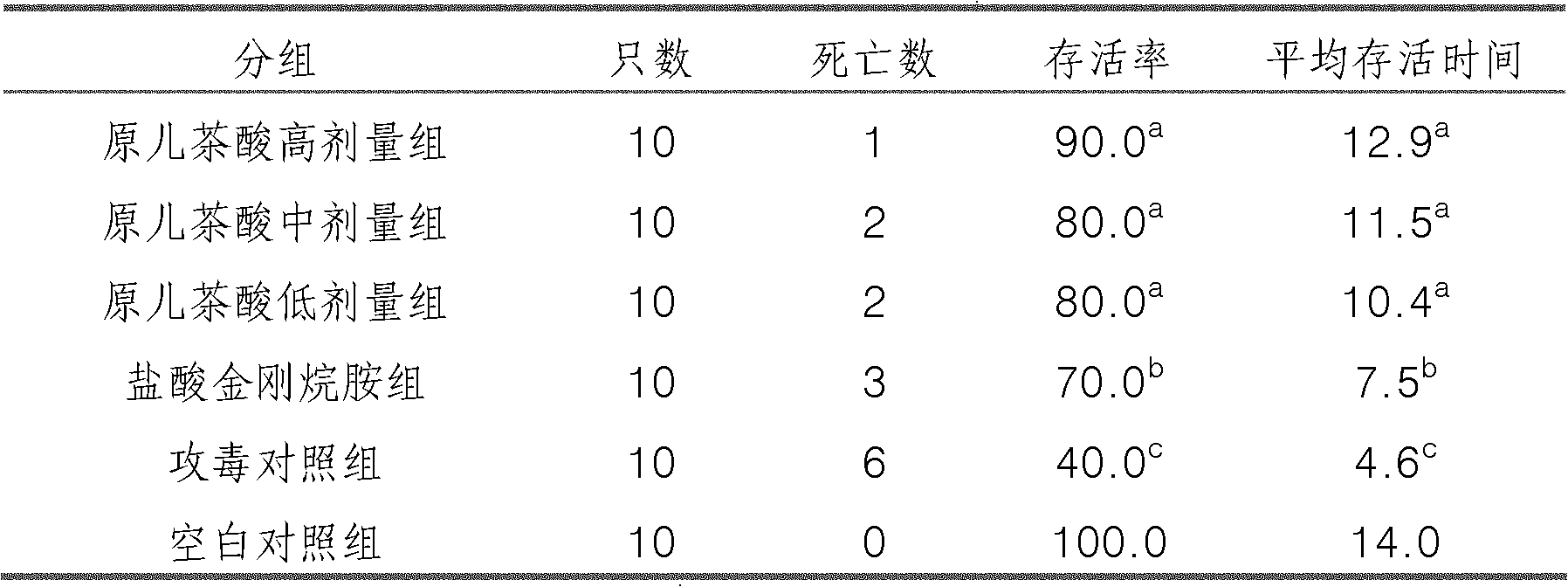
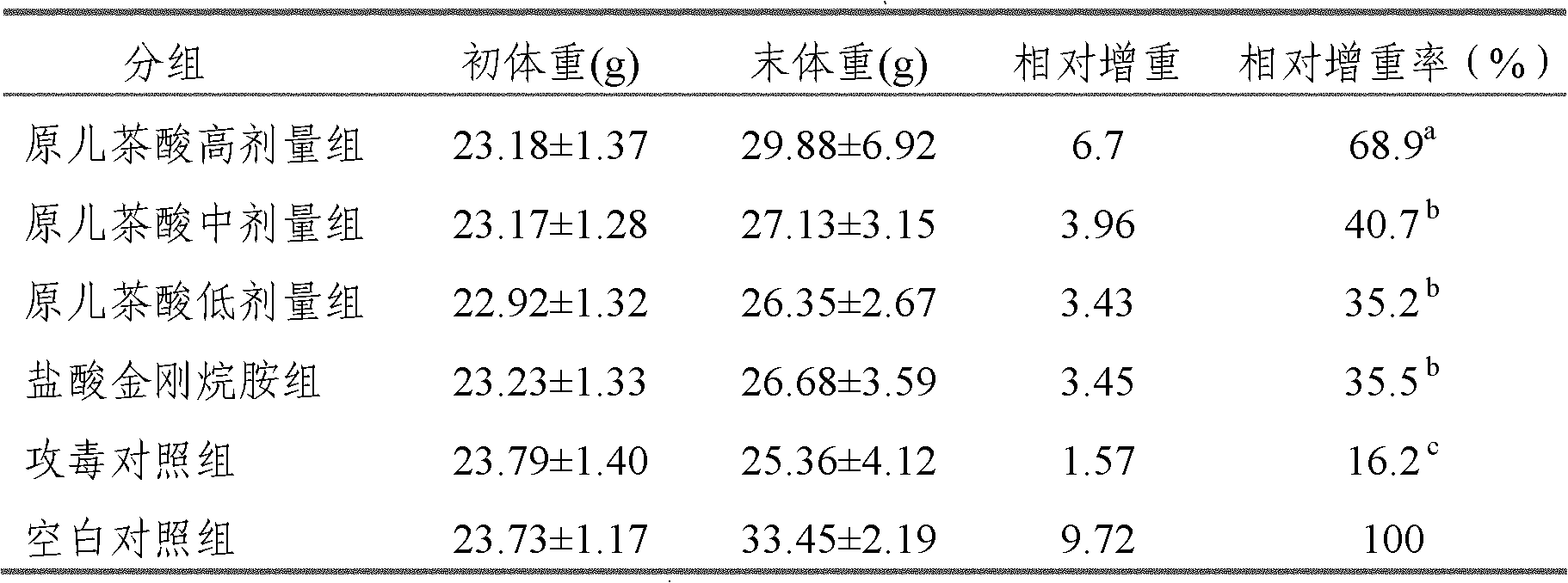
Application of protocatechuic acid in preparation of drugs for preventing and controlling livestock and poultry virus infectious diseases
ActiveCN102151256AReduce artificial infectionReduce mortalityOrganic active ingredientsPowder deliveryInfectious bronchitis virusAvian influenza virus
The invention provides an application of protocatechuic acid in the preparation of drugs for preventing and controlling livestock and poultry virus infectious diseases. The viruses include infectious bursal disease virus, avian influenza virus, infectious bronchitis virus and / or porcine transmissible gastroenteri tis virus. Meanwhile, the invention also relates to a protocatechuic acid preparation and a preparation method thereof.
Owner:CHINA AGRI UNIV
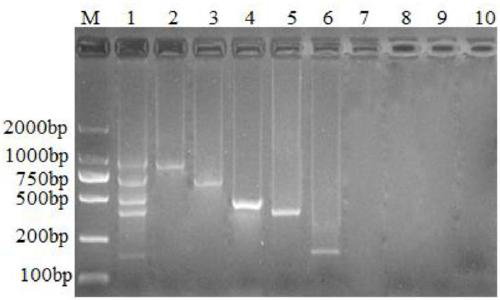
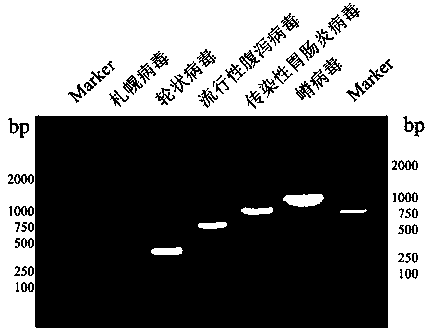
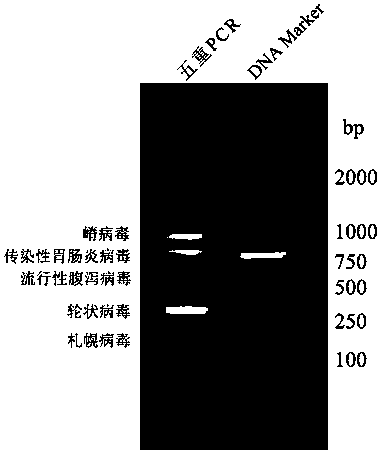
Method for simultaneously detecting multiple RT-PCR of GETV, PEDV, TGEV, PDCoV and PoRV
InactiveCN108950083AStrong specificityImprove efficiencyMicrobiological testing/measurementMicroorganism based processesRotavirus RNANucleotide
The invention discloses a multiple RT-PCR primer group for simultaneously detecting porcine gatahvirus (GETV), porcine epidemic diarrhea virus (PEDV), porcine deltacoronavirus (PDCoV) and porcine A rotaviruses (PoRV), which has a nucleotide sequence as shown in SEQ ID NO:1-SEQ ID NO:10. The invention further discloses a multiple RT-PCR detection method for detecting GETV, PEDV, TGEV, PDCoV and PoRV from a sample in one time by utilizing the multiple RT-PCR primer group. Compared with an existing conventional RT-PCR, the detection method has strong specificity and high sensitivity, can realizesimultaneous identification of five viruses including GETV, PEDV, TGEV, PDCoV and PoRV, and has accurate detection result and high detection efficiency.
Owner:HENAN AGRICULTURAL UNIVERSITY
Multiplex ligation-dependent probe amplification detection kit for simultaneously detecting five swine disease viruses, primers and probes
ActiveCN102943129AGuaranteed sensitivityGuaranteed specificityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseMultiplex ligation-dependent probe amplification
The invention discloses a multiplex ligation-dependent probe amplification detection kit for simultaneously detecting five swine disease viruses, primers and probes. The multiplex ligation-dependent probes are shown in sequence tables SEQ ID NO:1 to SEQ ID NO:10; and the primers are shown in sequence tables SEQ ID NO:11 to SEQ ID NO:12. By using the primers, the probes and / or the multiplex ligation-dependent probe amplification detection kit containing the primers and the probes, five important swine disease pathogens such as a swine influenza virus, a swine reproductive and respiratory syndrome virus, a pseudorabies virus, a swine transmissible gastroenteritis virus and a foot-and-mouth disease virus can be simultaneously detected, thereby saving the detection time and cost and being beneficial to accurately diagnosing the epidemic diseases in time.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
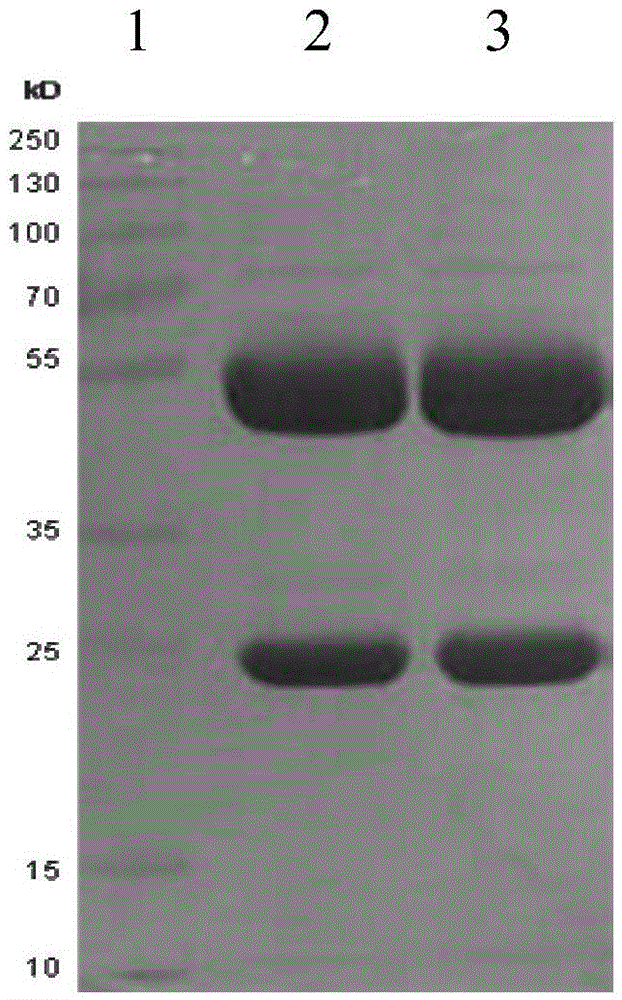
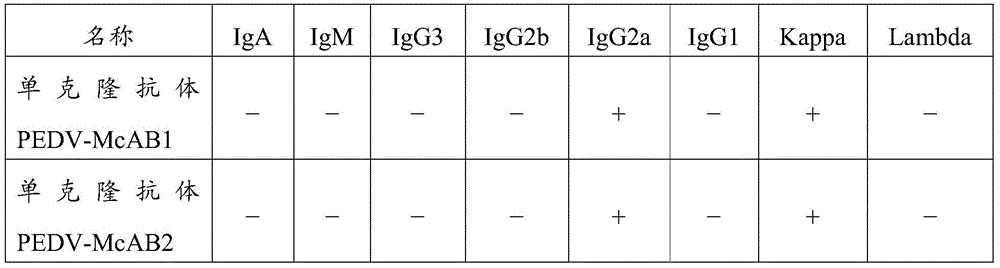
Detection kit and application thereof
ActiveCN105527437AHigh sensitivityBiological material analysisPorcine rotavirusPorcine Transmissible Gastroenteritis
The invention provides a detection kit containing two anti porcine epidemic diarrhea virus monoclonal antibodies PEDV-McAB1 and PEDV-McAB2, two anti porcine transmissible gastroenteritis virus monoclonal antibodies and two anti porcine rotavirus monoclonal antibodies; the kit can be applied for simultaneous detection of porcine epidemic diarrhea viruses, porcine transmissible gastroenteritis viruses and / or porcine rotaviruses with non-diagnostic purpose, moreover, the detection sensitivity of the kit for simultaneous detection of two or three kinds of viruses is higher than that of single detection of one kind of virus, and false positive results are avoided.
Owner:LUOYANG PULIKE WANTAI BIOTECH +2
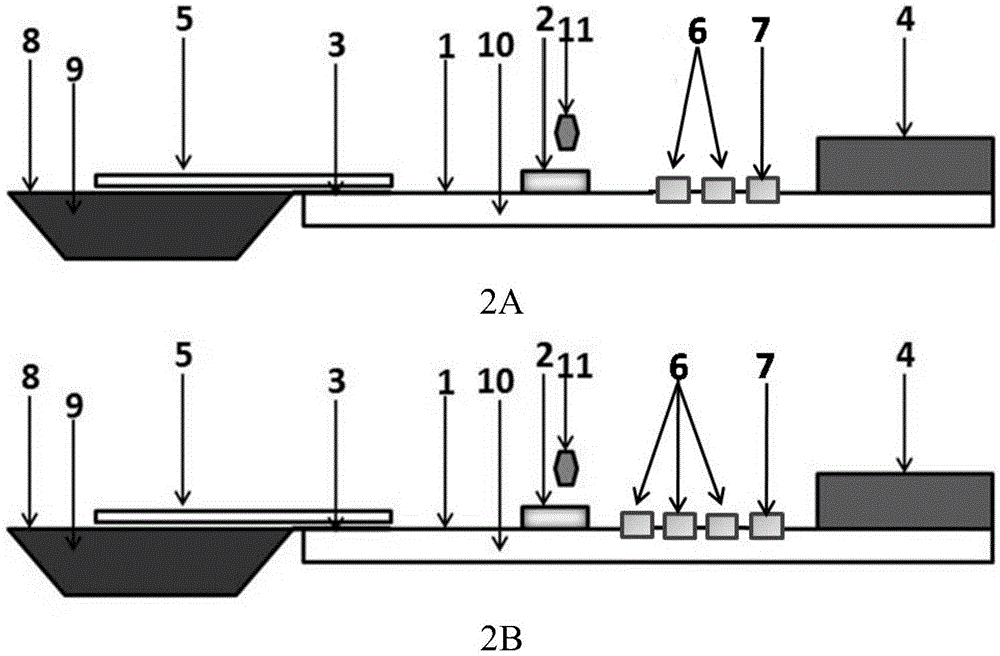
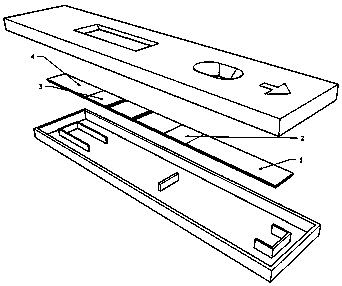
Test paper card for detecting swine transmissible gastroenteritis virus antibody and preparation and detection method of test paper card
InactiveCN107942061AImprove stabilityHigh sensitivityMaterial analysisCelluloseTransmissible gastroenteritis virus Antibody
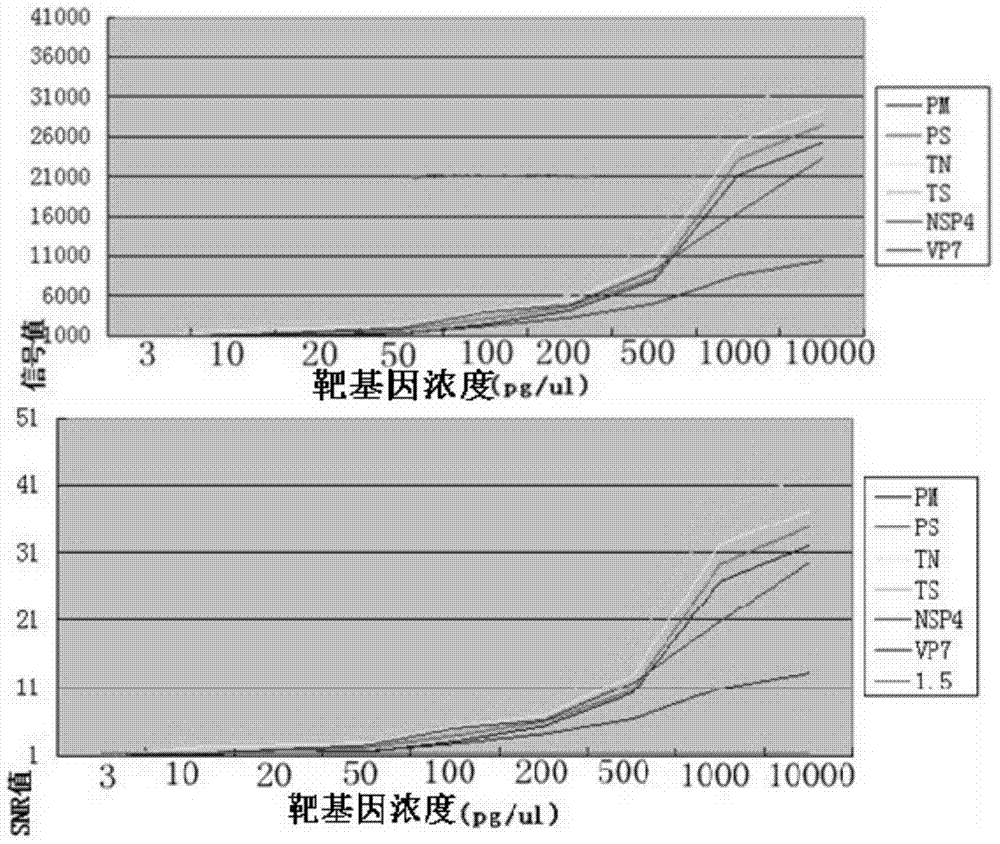
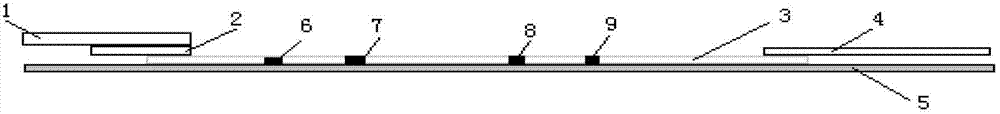
The invention relates to a test paper card for detecting porcine transmissible gastroenteritis virus antibody, preparation and detection method thereof, and belongs to the field of immunological detection. The test paper card includes a jammed case and a test strip, and the test strip includes a bottom plate and sequentially lapped and pasted on the Absorbent pad, detection pad, binding pad and sample pad on the base plate; the detection pad is a nitrocellulose membrane with a quality control line C and a detection line T, the quality control line C is coated with goat anti-mouse monoclonal antibody, and the detection line T is Coated with inactivated porcine transmissible gastroenteritis virus; the binding pad is a glass cellulose membrane embedded with time-resolved fluorescent microsphere-labeled anti-E2 protein monoclonal antibody; the sample pad is dried glass after soaking in the sample treatment solution Cellulose film. The test paper card prepared by the invention has better stability and higher sensitivity, and can achieve the purpose of semi-quantitative detection through fluorescence signal analysis.
Owner:洛阳现代生物技术研究院有限公司
TGEV and PEDV combined live vaccine and preparation method thereof
The invention discloses a combined live vaccine of transmissible gastroenteritis virus of swine (TGEV) and porcine epidemic diarrhea virus (PEDV) and a preparation method thereof. An attenuated swine transmissible gastroenteritis virus HB08 and an attenuated porcine epidemic diarrhea virus ZJ08 which are self-separated, attenuated and stored respectively undergo viral multiplication on ST cells and VeroE6 cells, and seedling and freeze drying are then carried out by adding a freeze-drying protective additive into a virus solution which is qualified after inspected. The two diseases, transmissible gastroenteritis of swine and porcine epidemic diarrhea virus, which are epidemic in clinic at present, can be effectively prevented by the use of the combined live vaccine.
Owner:兆丰华生物科技(南京)有限公司 +3
Molecular kit for rapidly identifying three types of piglet virus diarrhea and application of molecular kit
ActiveCN104611466ANo mutual interferenceLow minimum detectable concentrationMicrobiological testing/measurementMicroorganism based processesRotavirus RNAAstrovirus gastroenteritis
The invention discloses a kit for detecting pig epidemic diarrhea viruses, pig transmissible gastroenteritis viruses and pig rotaviruses. The kit comprises primer pairs shown by SEQ ID NO:1-2, SEQ ID NO:3-4 and SEQ ID NO:5-6 for respectively performing specific amplification on the pig epidemic diarrhea viruses, the pig transmissible gastroenteritis viruses and the pig rotaviruses. The invention also discloses applications of the primer pairs shown by the SEQ ID NO:1-2, the SEQ ID NO:3-4 and the SEQ ID NO:5-6 in preparation of reagents for detecting the pig epidemic diarrhea viruses, the pig transmissible gastroenteritis viruses and the pig rotaviruses. The detection kit disclosed by the invention can be used for accurately and effectively detecting the pig epidemic diarrhea viruses, the pig transmissible gastroenteritis viruses and the pig rotaviruses, and is strong in specificity, high in sensitivity, short in time consumption, rapid in detection and good in application prospect.
Owner:SICHUAN AGRI UNIV
Monoclonal antibody specially bound to TGEV (transmissible gastroenteritis of swine virus), pharmaceutical composition, kit and applications of monoclonal antibody, pharmaceutical composition and kit
ActiveCN109232736ABiological material analysisImmunoglobulins against virusesAstrovirus gastroenteritisMurine monoclonal antibody
The invention provides a monoclonal antibody specially bound to TGEV (transmissible gastroenteritis of swine virus) and a kit prepared from the monoclonal antibody. The TGEV can be determined rapidlyand accurately, and strains can be widely determined. Pharmaceutical composition prepared from the antibody can be used for broad-spectrum prevention and treatment of different TGEVs.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Method for preparing double yolk antibody of porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus
InactiveCN101845095AStrong specificityStrong targetingEgg immunoglobulinsImmunoglobulins against virusesPig farmsYolk
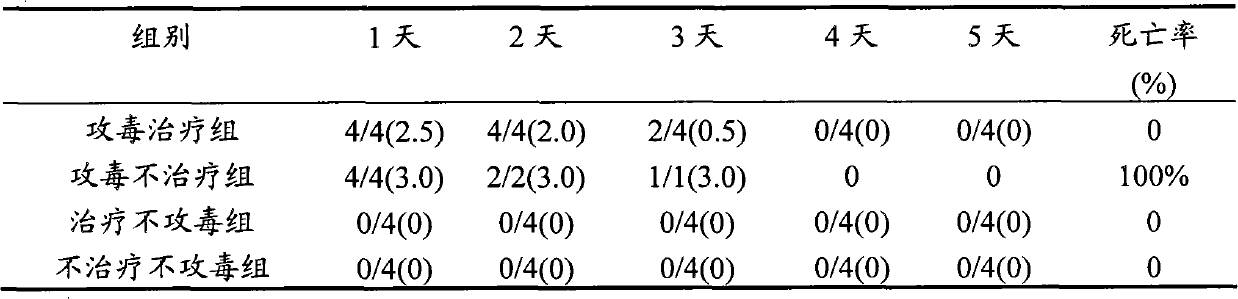
The invention discloses a method for preparing a double yolk antibody of a porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. The method comprises the following steps of: performing porcine transmissible gastroenteritis virus multiplication on porcine kidney cells (PK15); performing porcine epidemic diarrhea virus multiplication on African green monkey kidney cells (Vero); emulsifying the two cell cultures used as antigen with an oil emulsion adjuvant to prepare immunogen, namely, mixing the two kinds of viruses in a ratio of (1-3):(1-3) to prepare the immunogen; immunizing non-immunologic laying hens; and obtaining the double yolk antibody which can prevent and treat porcine transmissible gastroenteritis and porcine epidemic diarrhea based on the collection and purification of the yolk. When the double yolk antibody is used for curing experimental pigs, the clinical symptoms in the experiment are obviously reduced compared with a control group, and the death rate of the experimental group is obviously lower than that of the control group. The double yolk antibody has obvious preventing and treating functions when applied in a pig farm with high incidence rate of the porcine transmissible gastroenteritis and the porcine epidemic diarrhea.
Owner:PU LIKE BIO ENG
Multiplex RT-PCR detection primer for porcine delta coronavirus, porcine epidemic diarrhea virus and porcine transmissible gastroenteritis virus
ActiveCN105483291ARapid differential diagnosisMicrobiological testing/measurementMicroorganism based processesPorcine parvovirusBovine parvovirus
The invention discloses a multiplex RT-PCR detection primer for a porcine delta coronavirus, a porcine epidemic diarrhea virus and a porcine transmissible gastroenteritis virus. The minimum detection capacity of the multiplex RT-PCR for the three viruses is 4.05*10<1> copies / microliter, 4.52*10<3> copies / microliter and 5.47*10<3> copies / microliter respectively. The amplification results for a porcine parvovirus (PPV) and a porcine pseudorabies virus (PRV) are both negative. The multiplex RT-PCR detection results of 57 clinical samples show that one sample is infected with the three viruses at the same time, 11 samples are infected with the PDCoV, 15 samples are infected with the PEDV, one sample is infected with the TGEV, five samples are infected with the PDCoV and the PEDV, and one sample is infected with the PDCoV and the TGEV.
Owner:HENAN AGRICULTURAL UNIVERSITY
Multiplex PCR primer group for detecting porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus and porcine rotavirus simultaneously
ActiveCN103409558ANo cross reactionStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationPcr methodPorcine rotavirus
The invention discloses a multiplex PCR primer group for detecting porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus and porcine rotavirus simultaneously, and belongs to the field of virus detection. The primer group comprises three pairs of primers, wherein the primer sequences of the first pair of primers used for detecting the porcine epidemic diarrhea virus are respectively SEQ ID NO:1 and SEQ ID NO:2, the primer sequences of the second pair of primers used for detecting the porcine transmissible gastroenteritis virus are respectively SEQ ID NO:3 and SEQ ID NO:4, and the primer sequences of the third pair of primers used for detecting the porcine rotavirus are respectively SEQ ID NO:5 and SEQ ID NO:6. Through the application of the sequences of the primers, different strains of the porcine epidemic diarrhea virus, the porcine transmissible gastroenteritis virus and the porcine rotavirus can be detected simultaneously through the multiplex PCR method, and the detection results of the porcine epidemic diarrhea virus, the porcine transmissible gastroenteritis virus and the porcine rotavirus are masculine, while the defection results of other common pig-derived viruses are feminine, in conclusion, the primer group is strong in specificity, and good in repeatability; the PCR detection is carried out after the virus cDNA is subjected to gradient dilution, which shows that the sensitivity of the primers is high.
Owner:哈尔滨威科赛斯生物科技有限公司
Recombinant salmonella choleraesuis, bivalent genetic engineering vaccine and application
InactiveCN101880647AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacteriaBacteroidesRecombinant vaccines
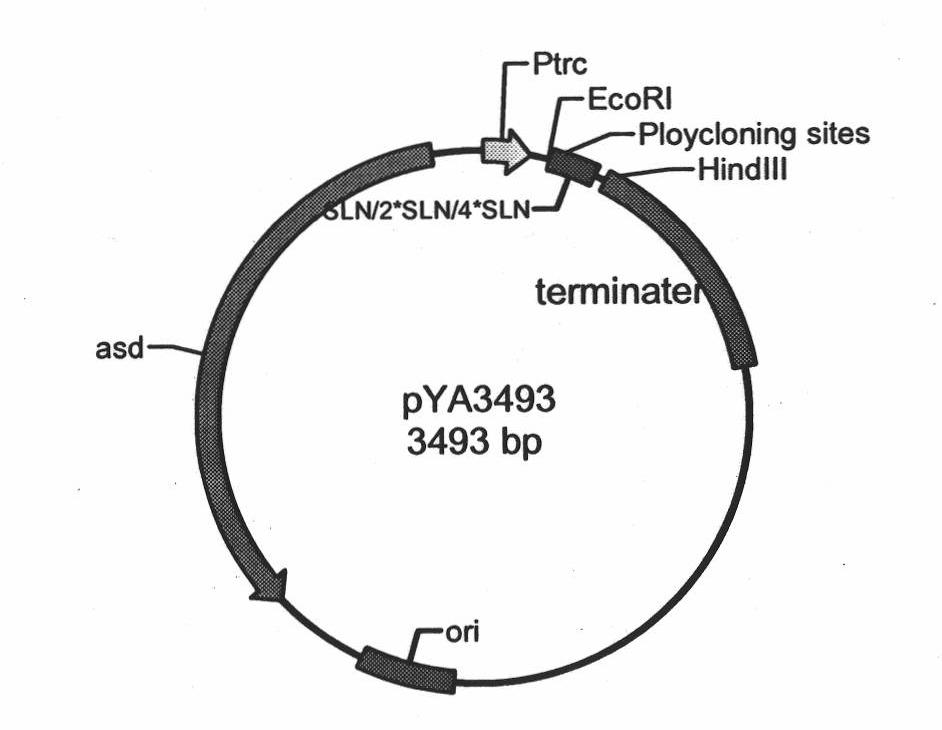
The invention belongs to the technical field of bacterial gene engineering of animals, and in particular relates to the construction of a recombinant salmonella choleraesuis strain which does not contain resistance markers and expresses major antigenic loci of porcine transmissible gastroenteritis virus, vaccine preparation and application. In the recombinant salmonella choleraesuis strain C500 (pYA-2SLN) which does not contain the resistance markers and expresses the major antigenic loci of the porcine transmissible gastroenteritis virus, the preservation No. is CCTCC NO: M 209189, and the strain misses asd genes which are necessary for the growth of salmonella choleraesuis and contains plasmid capable of expressing the asd genes, genes of an antigenic locus A, an antigenic locus D and an antigenic locus N321 of the porcine transmissible gastroenteritis virus in the strain. The invention also discloses a method for preparing the salmonella choleraesuis and porcine transmissible gastroenteritis vaccine by utilizing the recombinant strain and application thereof. The prepared recombinant vaccine can stimulate pigs to generate protective immune response for resisting the salmonella choleraesuis and the porcine transmissible gastroenteritis virus, and prevent the infection of the salmonella choleraesuis and the porcine transmissible gastroenteritis virus effectively.
Owner:HUAZHONG AGRI UNIV
Kit for detecting porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus and porcine rotavirus
ActiveCN104232801AEfficient detectionQuick checkMicrobiological testing/measurementMicroorganism based processesPorcine rotavirusVirology
The invention discloses a kit for detecting a porcine epidemic diarrhea virus, a porcine transmissible gastroenteritis virus and a porcine rotavirus. The gene detection kit disclosed by the invention can accurately and effectively detect the porcine epidemic diarrhea virus, the porcine transmissible gastroenteritis virus and the porcine rotavirus, and is strong in specificity, high in sensitivity, short in time consumption, fast in detection and good in application prospects.
Owner:SICHUAN AGRI UNIV
Colloidal gold strip for TGEV antibody and PEDV antibody
ActiveCN103033626AObvious superiorityGuaranteed FeaturesMaterial analysisSerum igeMonoclonal antibody
The invention discloses a test strip for quickly detecting a PEDV (Porcine Epidemic Diarrhea Virus) serum antibody and a TGEV (Transmissible Gastroenteritis Virus) serum antibody and a preparation method of the test strip. The test strip comprises a support layer, a sample loading layer, a gold labeling protein release pad, a detection layer and an absorption layer, wherein a gold labeling protein and a detection line protein of the test strip are an S1 protein of a PEDV and a recombination protein of a TGEV, which are obtained by an efficient prokaryotic expression system; the recombination protein comprises an S protein AD site; and two quality control line proteins are monoclonal antibodies for the two proteins. Compared with the traditional test strip for detecting the PEDV antibody and the TGEV antibody, the test strip is high in specificity and safety and simple to operate, and judges results quickly.
Owner:兆丰华生物科技(南京)有限公司 +3
DPO (Dual Priming Oligonucleotide) primer group for porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus and porcine rotavirus detection and application of DPO primer group
ActiveCN108060269ALarge annealing temperature rangeStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationReal-Time PCRsPorcine rotavirus
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Quintuple RT-PCR detection kit for porcine viral diarrhea viruses
ActiveCN105506182AAccurate judgmentRapid diagnosisMicrobiological testing/measurementMicroorganism based processesTotal rnaPorcine rotavirus
The invention discloses a quintuple RT-PCR detection method and kit for porcine epidemic diarrhea viruses, porcine transmissible gastroenteritis viruses, porcine rotaviruses, porcine sapoviruses and porcine kobuviruses. The kit comprises ten specificity amplification primers. In the using process of the kit, total RNA of a sample to be detected is subjected to reverse transcription to become cDNA through a 6-basic-group random primer, a detection reaction system in the kit is used for RCR amplification with the cDNA as a template, and whether the sample to be detected is infected with one kind of pathogens or is under mixed infection is determined according to different sizes of amplified PCR segments of different pathogens. On the condition of ensuring specificity and sensitivity, the kit has the advantages of being easy and quick to operate and lowering detection cost and labor intensity, and is suitable for field sample detection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
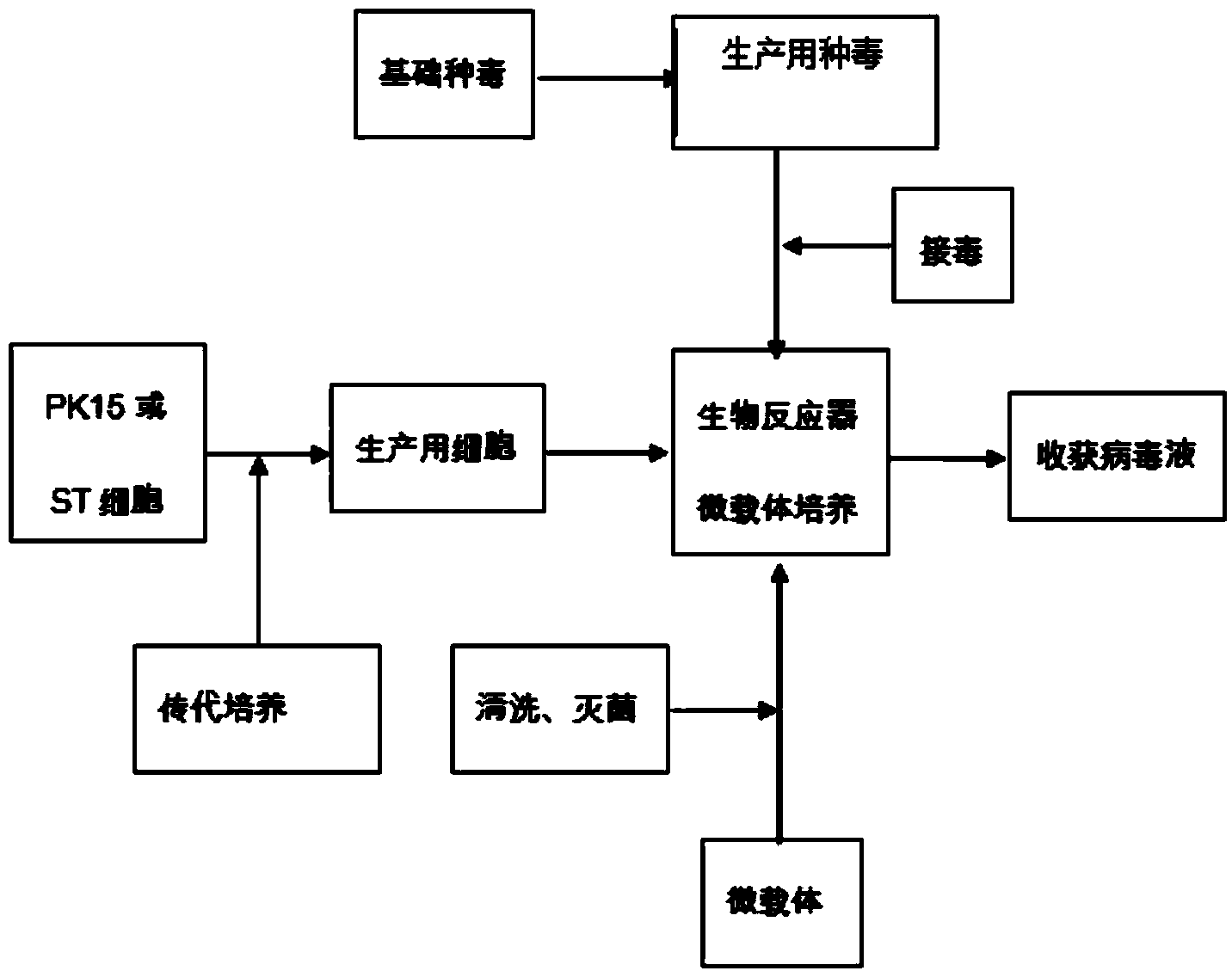
Production method of transmissible gastroenteritis virus vaccine
ActiveCN103550771AHigh potencyReduce manufacturing costMicroorganism based processesAntiviralsViral VaccineBottle
The invention discloses a production method of a transmissible gastroenteritis virus vaccine. The production method comprises the steps of carrying out subculture on cells for production so as to form monolayer cells; multiplying virus seeds for production, namely inoculating the basic virus seeds to the monolayer cells for culturing; dissociating the monolayer cells into a monolayer cell suspension liquid, and inoculating the cell suspension liquid into a bioreactor for culturing; multiplying vaccine culturing virus liquid, namely inoculating the virus seeds for production to the cells to be cultured after the quantity of the cells reaches 5*106-5*107 unit / ml; and harvesting the virus liquid. The production method has the advantages that the production cost can be greatly lowered, the production cycles are short, each production cycle only lasts for 5-7 days, and compared with that of transmissible gastroenteritis virus generated by an existing spinner bottle culture method, the titer of the transmissible gastroenteritis virus produced by the method is higher; the automation degree is high, few workers are required, the production process is simple and stable, the operation is easy, the yield is high, the occupied area is small, the production scale can be easily and rapidly expanded, and the quality is balanced and stable basically; the environmental pollution is slight and is easy to avoid.
Owner:成都史纪生物制药有限公司
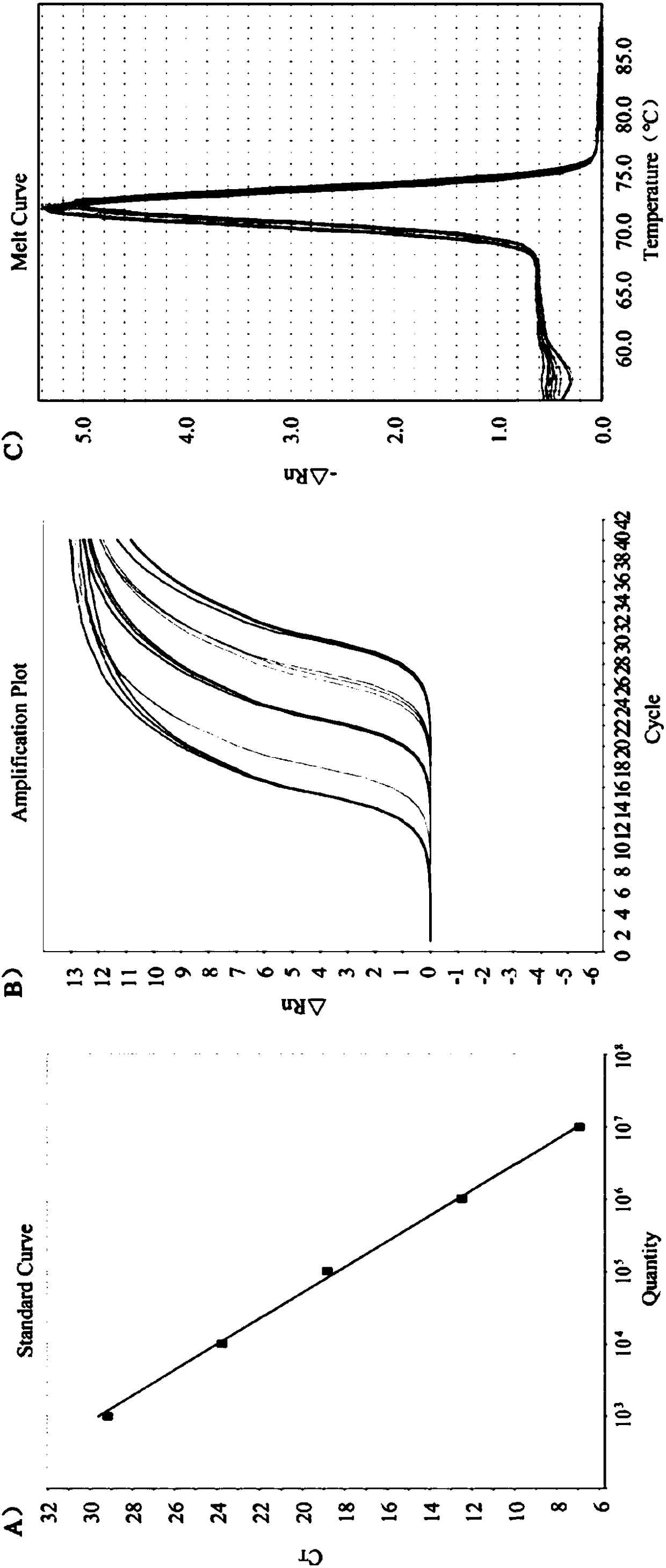
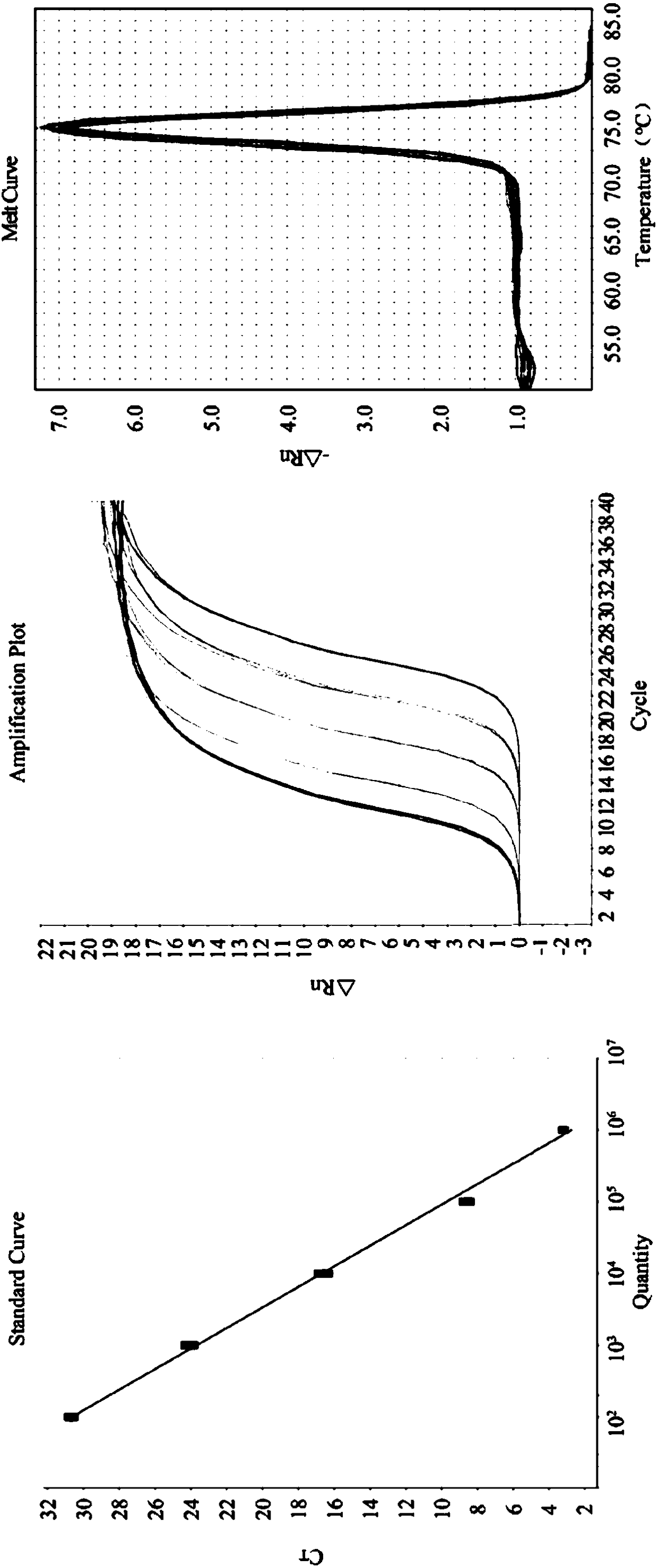
Double microdroplet digital PCR (Polymerase Chain Reaction) absolute quantitative detection kit for porcine epidemic diarrhea virus and transmissible gastroenteritis virus
InactiveCN105543416AImprove efficiencySimple and fast operationMicrobiological testing/measurementDNA/RNA fragmentationEpidemiologic surveyTransmissible gastroenteritis virus
The invention provides a specific primer and probe combination for detecting porcine epidemic diarrhea virus (PEDV) and transmissible gastroenteritis virus (TGEV). The specific primer and probe combination comprises two pairs of specific primers and two specific probes used in conjunction with the primers. The invention provides a detection kit or a detection reagent for detecting PEDV and TGEV at the same time. The specific primer and the detection kit or the detection reagent provided by the invention have the advantages of rapidness, sensitivity, specificity and the like, and can lay a foundation for double digital PCR (Polymerase Chain Reaction) absolute quantitative detection of PEDV and TEGV, epidemiological investigation, vaccine usage and the like.
Owner:BEIJING SEAGULL BIOVENTURES & BIOTECH CO LTD
Multiple reverse transcription polymerase chain reaction detection method for swine transmissible gastroenteritis
InactiveCN102586409AIncreased sensitivityStrong specificityMicrobiological testing/measurementSwine Transmissible GastroenteritisPorcine epidemic diarrhoea virus
The invention relates to a multiple reverse transcription polymerase chain reaction detection method for swine transmissible gastroenteritis. An antibody is needed to be prepared by an euzymelinked immunosorbent assay, so that the period is longer, and external factors have larger interference on the diagnostic method of the euzymelinked immunosorbent assay. The method comprises the following steps of: designing and synthesizing a transmissible swine gastroenteritis virus gene primer; establishing a multiple reverse transcription polymerase chain reaction diagnostic method of the transmissible swine gastroenteritis viruses and porcine epidemic diarrhea viruses; optimizing concentration of the primer of a bigeminy reverse transcription polymerase chain reaction; optimizing the concentration of magnesium oxide of the bigeminy reverse transcription polymerase chain reaction; optimizing the concentration of triphosphoric acid base deoxynucleotide of the bigeminy reverse transcription polymerase chain reaction; determining the optimal annealing temperature of the bigeminy reverse transcription polymerase chain reaction; testing the sensitivity of the bigeminy reverse transcription polymerase chain reaction; and testing the specificity of the bigeminy reverse transcription polymerase chain reaction. The multiple reverse transcription polymerase chain reaction detection method for swine transmissible gastroenteritis is suitable for detecting swine transmissible gastroenteritis.
Owner:郑世民
Primer and multiple RT-PCR method for identifying and detecting swine diarrhea coronavirus
InactiveCN107557494AEfficient detection methodShorten detection timeMicrobiological testing/measurementMicroorganism based processesEpidemic diarrheaSwine Transmissible Gastroenteritis
Owner:HUAZHONG AGRI UNIV
Production method of porcine transmissible gastroenteritis virus by utilizing bioreactor
ActiveCN102660510AQuality improvementIncrease productionMicroorganism based processesViruses/bacteriophagesVultureTiter
The invention provides a production method of porcine transmissible gastroenteritis virus by utilizing a bioreactor. The method comprises the following steps: 1) preparing monolayer subculture cells; 2) preparing virus seed for porcine transmissible gastroenteritis virus production; 3) preparing a cell suspension from the monolayer subculture cells prepared in the step 1), and inoculating into a bioreactor to carry out adsorption culture on the subculture cells in a microcarrier in the bioreactor; 4) inoculating the virus seed prepared in the step 2) at the inoculation amount of 2 to 5 percent when the subculture cells grow to 80-90 percent of the microcarrier, the empty bead rate is lower than 5 percent, the full bead rate is more than 80 percent and the cell count is over 3-5*10<6> per mL, and performing virus adsorption culture; and 5) harvesting virus fluid when over 80 percent of the subculture cells on the microcarrier have pathological changes. The method can be used for solving the problems of low production efficiency, unstable product quality and low virus titer, so that the unit vulture titer of the virus can be improved by 5 to 10 times, the quality and yield of vaccine can be comprehensively improved, and the safety of vaccine is improved.
Owner:兆丰华生物科技(南京)有限公司 +1
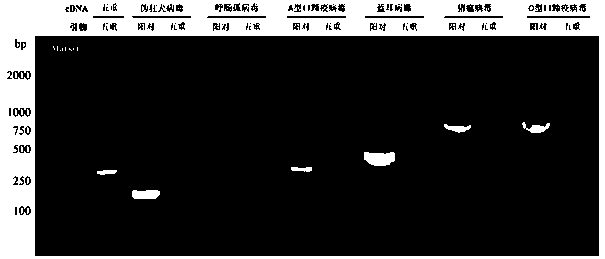
Fourfold RT-PCR detection primer and reagent kit of four porcine epidemic diarrheaviruses
ActiveCN110093461AStrong specificityRepeatableMicrobiological testing/measurementMicroorganism based processesEpidemic diarrheaAstrovirus gastroenteritis
The invention relates to a detection primer and reagent kit of four RT-PCR of porcine epidemic diarrheaviruses, transmissible gastroenteritis of swine, porcine rotaviruses and porcine deltacoronaviruses, and belongs to the technical field of molecular detection. The fourfold RT-PCR detection primer comprises detection primers PEDV F and PEDV R of porcine epidemic diarrhea viruses, detection primers TGEV F and TGEV R of the transmissible gastroenteritis of swine, detection primers PoRV F and PoRV R of the porcine rotaviruses and detection primers PDCoV-F and PDCoV-R of the porcine delta coronaviruses. The primer is high in specificity, has repeatability, is high in sensitivity and is high in clinical reliability.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com