Recombinant salmonella choleraesuis, bivalent genetic engineering vaccine and application
A technology for Salmonella and cholera suis, which is applied in the field of construction of recombinant Salmonella cholera suis live vaccine strains, can solve the problems of biosafety, unacceptable, large economic losses, etc., and achieves good biosafety and good immune protection. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Synthesis of Gene Fragment Containing Porcine Transmissible Gastroenteritis Virus Main Antigen Site
[0049] 1. Selection of main antigenic sites
[0050] The S protein of porcine transmissible gastroenteritis virus contains four antigenic sites, A, B, C, and D, among which the A and D antigenic sites play an important role in inducing neutralizing antibodies, and the A antigenic site is a major B cell antigen epitopes. Porcine transmissible gastroenteritis virus contains 4 main T cell epitopes, of which the N on the N protein 321 (321-335 residue region) cell antigen site can be the strongest T cell response, and can cooperate with B cell epitopes of different proteins in TGEV.
[0051] 2. Design of S and N gene primers and comparison of their homology
[0052] Two pairs of primers were designed with reference to the reported gene sequences of porcine transmissible gastroenteritis virus S and N (GenBank No: DQ443743), and S gene and N gene. The size of t...
Embodiment 2
[0061] Construction of embodiment 2 recombinant plasmid pYA-2SLN
[0062] 1. Cloning of SLN gene
[0063] The recombinant bacteria (DH5α / pET-2SLN) containing the SLN gene sequence were transferred to a bacterial bottle containing 3 mL of LB medium at a volume ratio of 1:100, and cultured overnight at 37°C and 200 rpm / min for 8 hours. According to the instructions of the bacterial plasmid extraction kit (purchased from Beijing BioFlux Company), the plasmid was extracted as a template for restriction digestion.
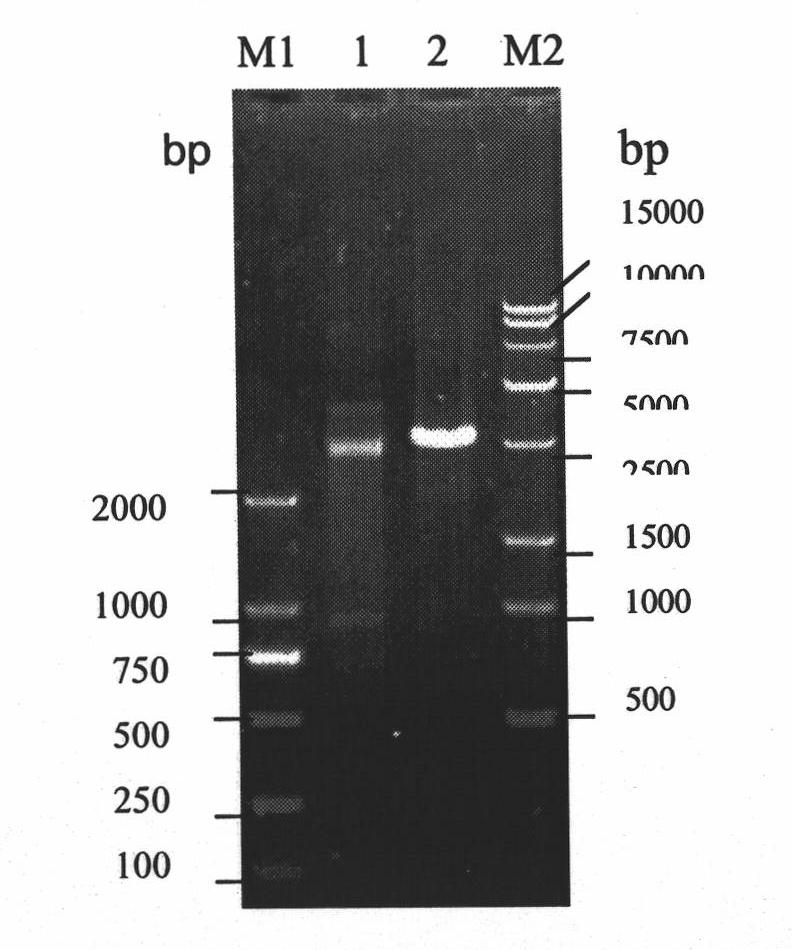
[0064] The SLN and pET-28a vectors (purchased from Treasure Bioengineering (Dalian) Co., Ltd.) were digested with EcoRI and NotI, recovered and purified, then ligated with T4DNA ligase, and then transformed into DH5α competent bacteria in a water bath at 16°C for 12h, and cultured at 37°C for 12h. Pick the bacteria into the liquid LB medium, shake and culture at 37°C and 200rpm / min for 12h, prepare a small amount of plasmids to obtain the plasmid pET-SLN (see Figure ...
Embodiment 3
[0076] Example 3 Construction of the Salmonella choleraesuis C500 / pYA-2SLN recombinant strain expressing the SLN gene fragment
[0077] The recombinant shuttle plasmid pYA-2SLN (see Example 2 for the source) was electrotransformed (parameters: voltage 2.0KV, time 4ms, capacitance 25μF and pulse resistance 200 ohms) to the C500ΔcrpΔasd deletion strain of Salmonella choleraesuis, and positive screening was performed on the DAP negative plate Cloning, picking a single colony for culture, and using primers pYASLNI / pYASLNII for PCR identification (PCR reaction system (25μL): 10×Buffer 2.5μL, 25mmol / L MgCl 2 2 μL, 2 μmol / L dNTPs 1 μL, 10 μg / mL upstream and downstream primers 1 μL each, 2U / μL Taq DNA polymerase 0.5 μL, sterile water 16 μL, template 1 μL. PCR reaction conditions: denaturation at 94°C for 4 minutes; then 25 cycles of 30s at 94°C, 30s at 56°C, and 60s at 72°C; and extension at 72°C for 10 minutes. PCR products were separated by 80V electrophoresis on 1.0% agarose gel,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com