TGEV and PEDV combined live vaccine and preparation method thereof
A technology of live vaccines and viruses, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve the problems of poor protection against swine diarrhea, unsatisfactory effects, and economic losses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Attenuated strain source
[0033] 1. Attenuated HB08 strain of porcine transmissible gastroenteritis virus
[0034] Take transmissible gastroenteritis antigen positive disease material from a pig farm in Hebei, add PBS according to the ratio of 1:5 (weight: volume), freeze and thaw repeatedly 3 times, centrifuge to get supernatant, filter with 0.22μm filter membrane, add to the filtrate Trypsin with a final concentration of 20 μg / ml was treated at 37° C. for 1.5 hours.
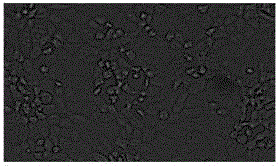
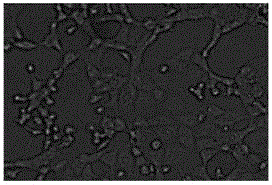
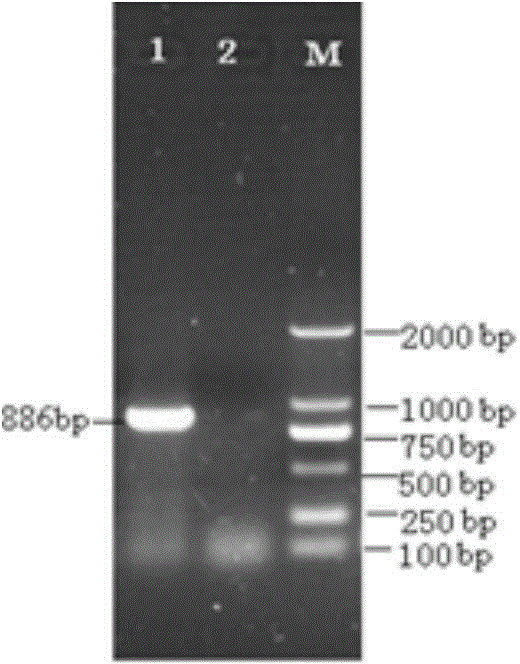
[0035] A monolayer of ST cells was inoculated according to conventional methods, and blank cells were set as controls. Significant and stable CPE changes appeared at the 17th generation ( figure 1 ). Design the detection primer pair according to the S gene: S-F: 5'-TATTTGTGGTYTTGGTYGTAATGC-3'; S-R: 5'-GGCTGTTTGGTAACTAATTTRCCA-3', perform 32 cycles at 95°C 45S, 50°C 45S, 72°C for 1min, then extend at 72°C for 5min . The amplified S gene fragment is 886bp, see the results figure 2 .
[0...
Embodiment 2
[0055] Embodiment 2 attenuated strain characteristic
[0056] 1. Attenuated HB08 strain of porcine transmissible gastroenteritis virus
[0057] The 125th and 165th passage viruses were diluted to 10 with DMEM respectively. 3.0 TCID 50 / ml, mixed with an equal amount of TGEV-specific serum, neutralized at 37°C for 1 hour, inoculated into 6 wells of a 96-well cell plate covered with a single layer of ST cells, and set 6 wells of non-neutralizing virus positive control cells and only Inoculate 6 wells of the negative control cells in the cell maintenance solution, culture in a 5% CO2 incubator at 37°C for 72-120 hours, and observe the cytopathic changes. Results The cells in the test group had no pathological changes, while the virus control group showed typical CPE changes.
[0058] The 145th generation seed virus of the attenuated porcine transmissible gastroenteritis virus HB08 strain was inoculated with 10 7.0 TCID 50 Inoculate 3 healthy piglets at the age of 3 at a dos...
Embodiment 3
[0067] Example 3 Preparation of dual vaccine
[0068] 1. Preparation of virus liquid for seedling production
[0069] Inoculate the well-growing monolayer cells ST and Vero E6 with the 155th generation of the attenuated porcine transmissible gastroenteritis virus strain HB08 and the 135th generation of the attenuated porcine epidemic diarrhea virus strain ZJ08, discard the cell growth medium, and follow the 2% After inoculation at 37°C for 1 hour, replenish the cell growth maintenance solution and continue culturing. When the CPE reached 80%, the virus was harvested, frozen and thawed 3 times at -20°C / room temperature, and the same batch of viruses were mixed together. Two batches of vaccine virus liquids were prepared by this method, and samples were taken for sterility test, mycoplasma test, exogenous virus test and determination of virus content at the same time. Choose virus content ≥ 10 6.0 TCID 50 / ml of virus liquid is used for seedling production.
[0070] 2 Mixin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com