Fusion protein of SARS-CoV-2, and vaccine composition of fusion protein
A vaccine composition, fusion protein technology, applied in the directions of fusion polypeptides, microorganisms, hybrid peptides, etc., can solve the problems of short duration of immune response, multiple vaccinations, poor immunogenicity, etc. The effect of suppressing replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation, identification and content determination of fusion protein RBD-mFc
[0033] According to NCBI (https: / / www.ncbi.nlm.nih.gov) the gene sequence (amino acid sequence such as SEQ ID No. 1), and the gene sequence of the Fc protein in mouse immunoglobulin IgG (the amino acid sequence is shown in SEQ ID No.2), and the fusion protein was prepared by genetic engineering.
[0034] (1) Cloning construction and identification of COVID-19 antigenic protein RBD and mFc fusion protein
[0035]Fully synthesize the nucleotide sequence of RBD-mFc (the nucleotide sequence is shown in SEQ ID No.4, the amino acid sequence is shown in SEQ ID No.3), and introduce specific BamHI and XhoI restriction enzyme cutting sites, the mammalian cell expression vector PCDNA3.1 and RBD-mFc gene, double digestion with BamHI and XhoI at the same time, after digestion, perform 10g / L agarose gel electrophoresis, and pass through the gel Recovery and purification kit to purify the di...
Embodiment 2
[0042] Embodiment 2: the preparation of subunit vaccine
[0043] (1) Dilute the RBD-mFc fusion protein prepared in Example 1 with PBS buffered saline solution, and mix thoroughly to obtain the vaccine RBD-mFc with a recombinant fusion protein content of 10 μg / mL.
[0044] (2) Dilute the RBD-mFc fusion protein prepared in Example 1 with PBS buffered saline solution, and add aluminum adjuvant to mix thoroughly to obtain a vaccine composition RBD-mFc / Al;
[0045] (3) Dilute the RBD-mFc fusion protein prepared in Example 1 with PBS buffered saline solution, add complete Freund's adjuvant, stir to make it a white emulsion, and obtain a recombinant fusion protein with a content of 10 μg / mL. Vaccine composition complete Freund's adjuvant RBD-mFc / FA (RBD-mFc / CFA); use PBS buffered saline solution to dilute the RBD-mFc fusion protein prepared in Example 1, add incomplete Freund's adjuvant, stir Make it into a white emulsion, that is, obtain a vaccine composition incomplete Freund's ...
Embodiment 3
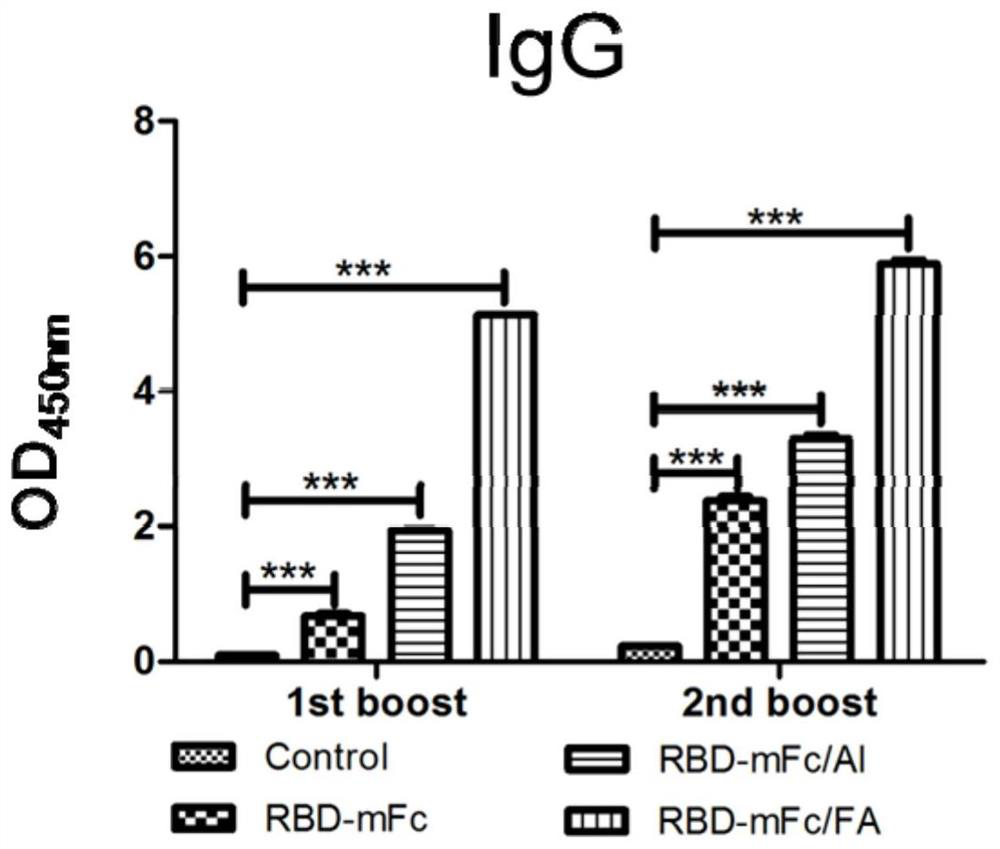
[0046] Example 3: ELISA immunoassay
[0047] (1) Immunization of mice
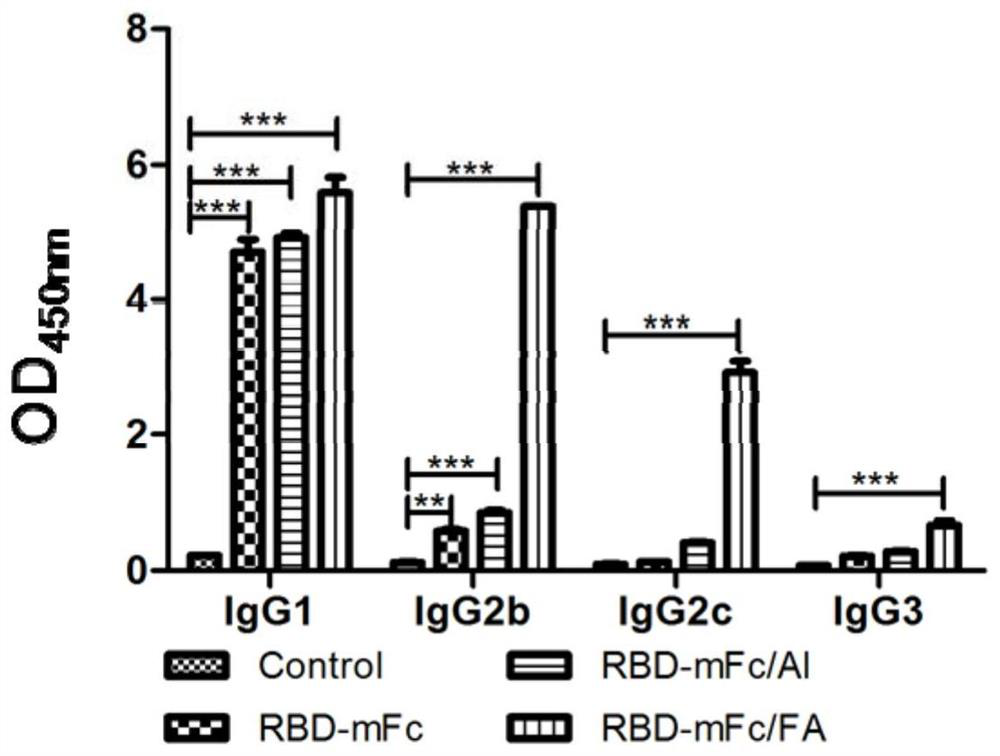
[0048] Twenty C57BL / 6 mice aged 6-8 weeks were randomly divided into 4 groups, 5 mice in each group, and the prepared vaccine was injected on the 0th and 28th day with the first initial immunization and the second booster immunization scheme, respectively. Only 0.1mL per injection.
[0049] RBD-mFc group: intramuscular injection of the vaccine RBD-mFc prepared in Example 2 on days 0 and 28;
[0050] RBD-mFc / Al group: intramuscular injection of the vaccine composition RBD-mFc / Al prepared in Example 2 on days 0 and 28;
[0051] RBD-mFc / FA group: on the 0th day, the vaccine composition prepared in Example 2 was injected intramuscularly with complete Freund's adjuvant RBD-mFc / FA (RBD-mFc / CFA); on the 28th day, the vaccine composition prepared in Example 2 was injected intramuscularly Vaccine composition Incomplete Freund's adjuvant RBD-mFc / FA (RBD-mFc / IFA).
[0052] Control group: a blank control group, in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com