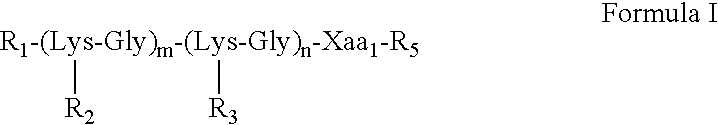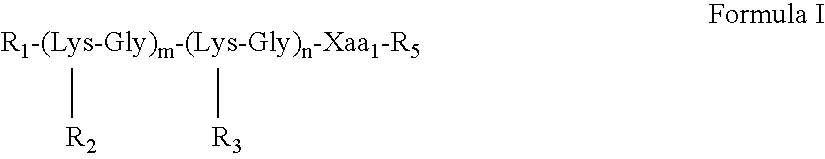Patents
Literature
33 results about "Core peptide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods and composition for derepressions of IAP-inhibited caspase
InactiveUS20060258581A1Promote apoptosisReduce severityCompound screeningApoptosis detectionDerepressionCaspase
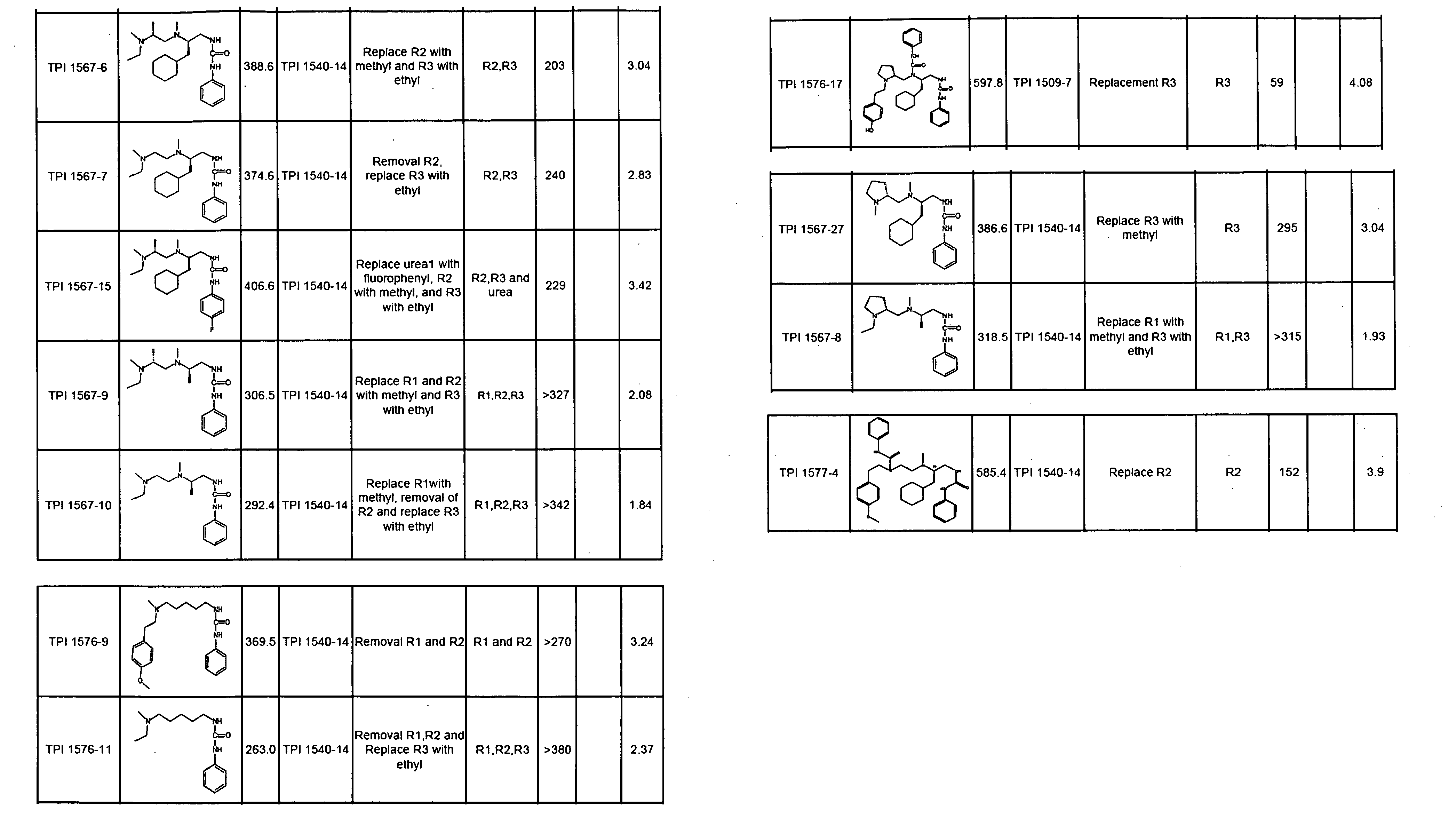
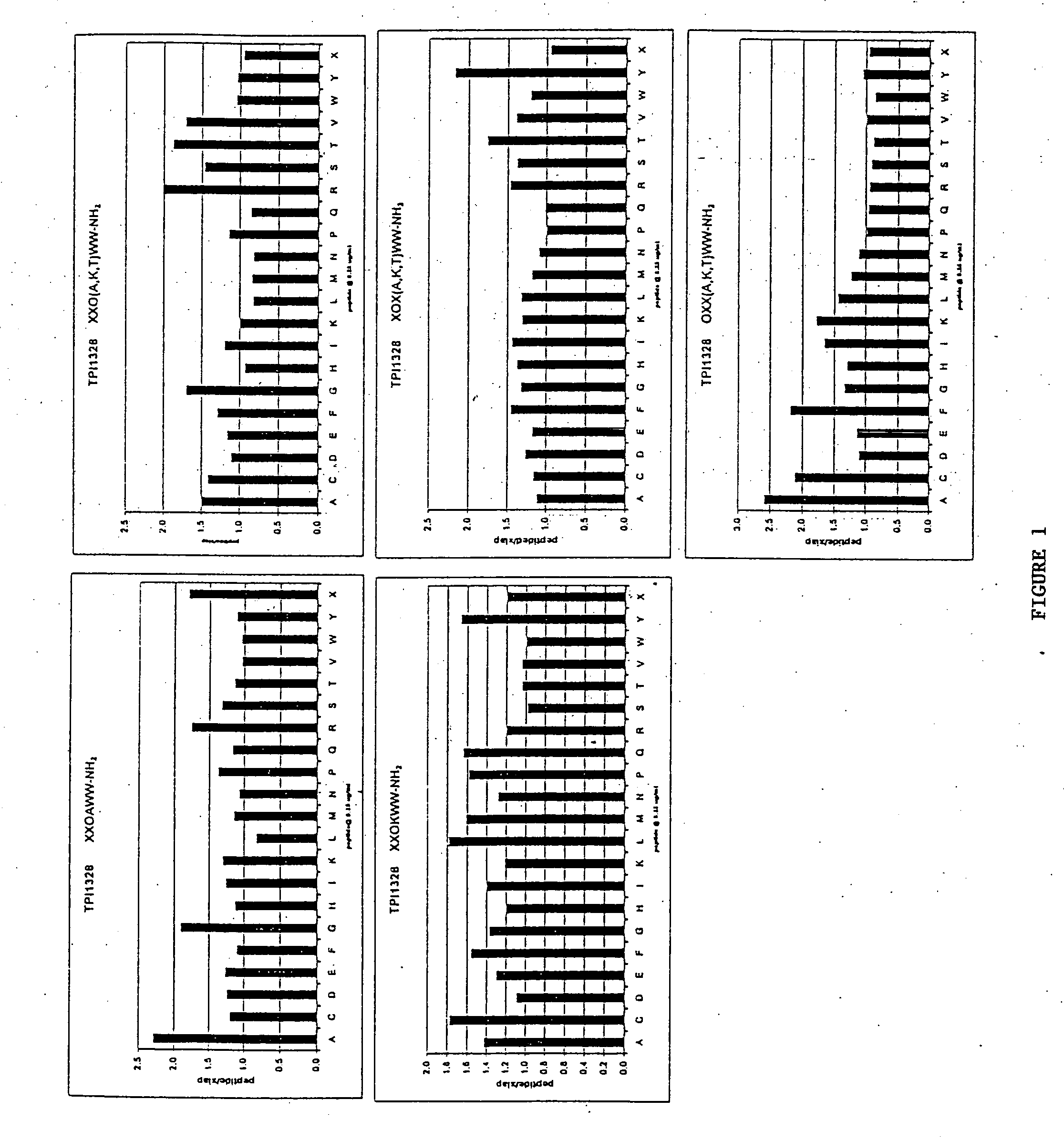
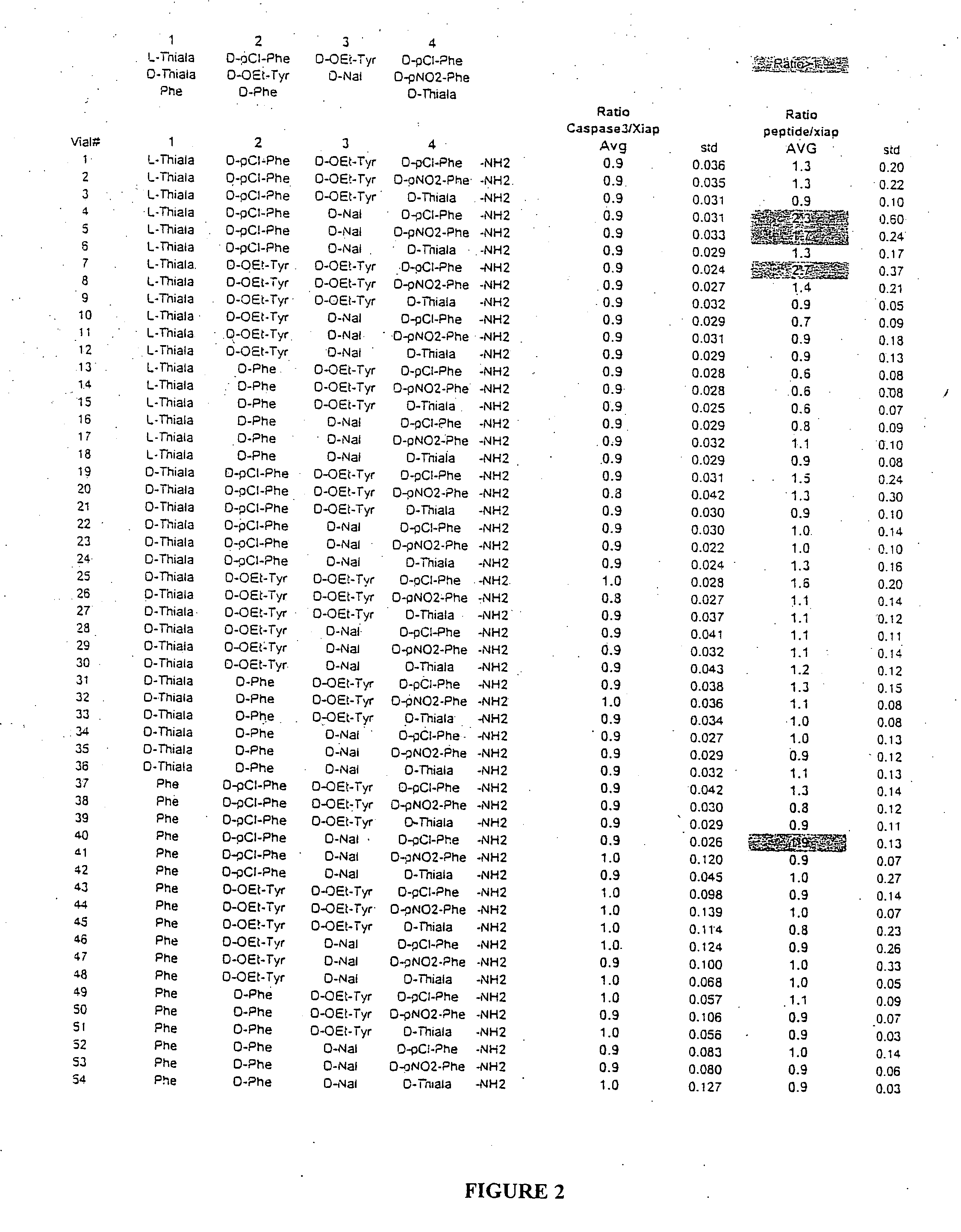
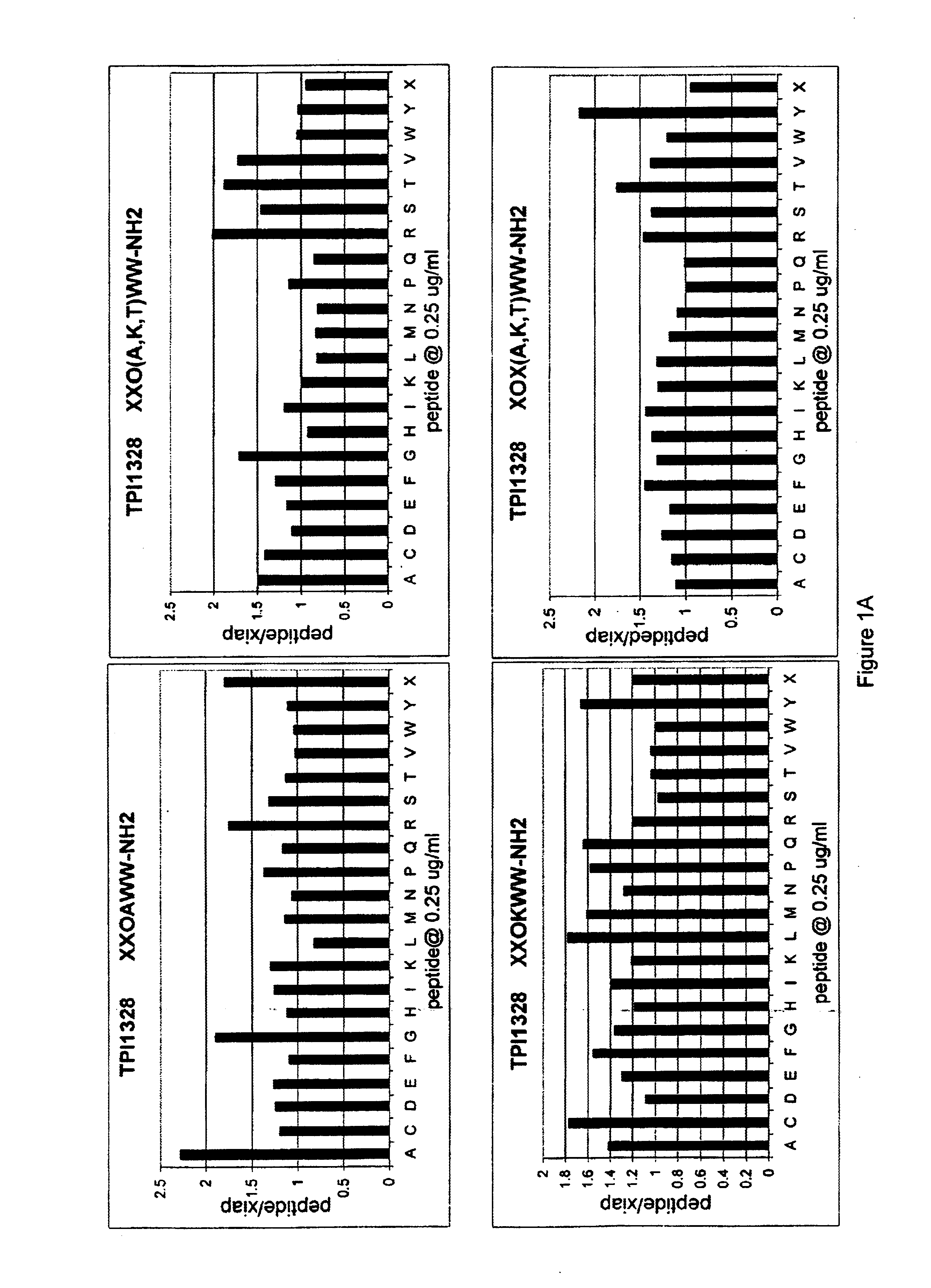
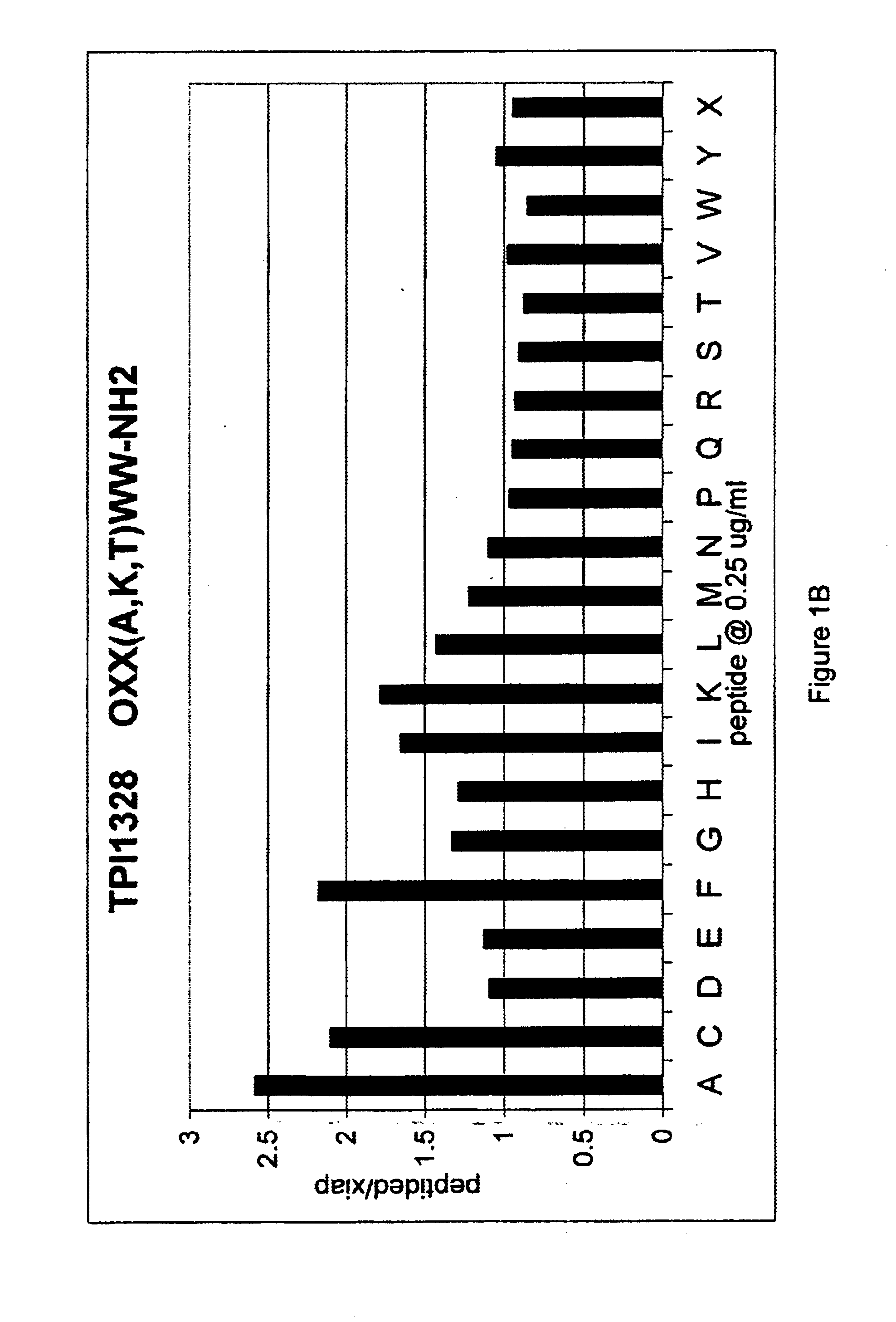
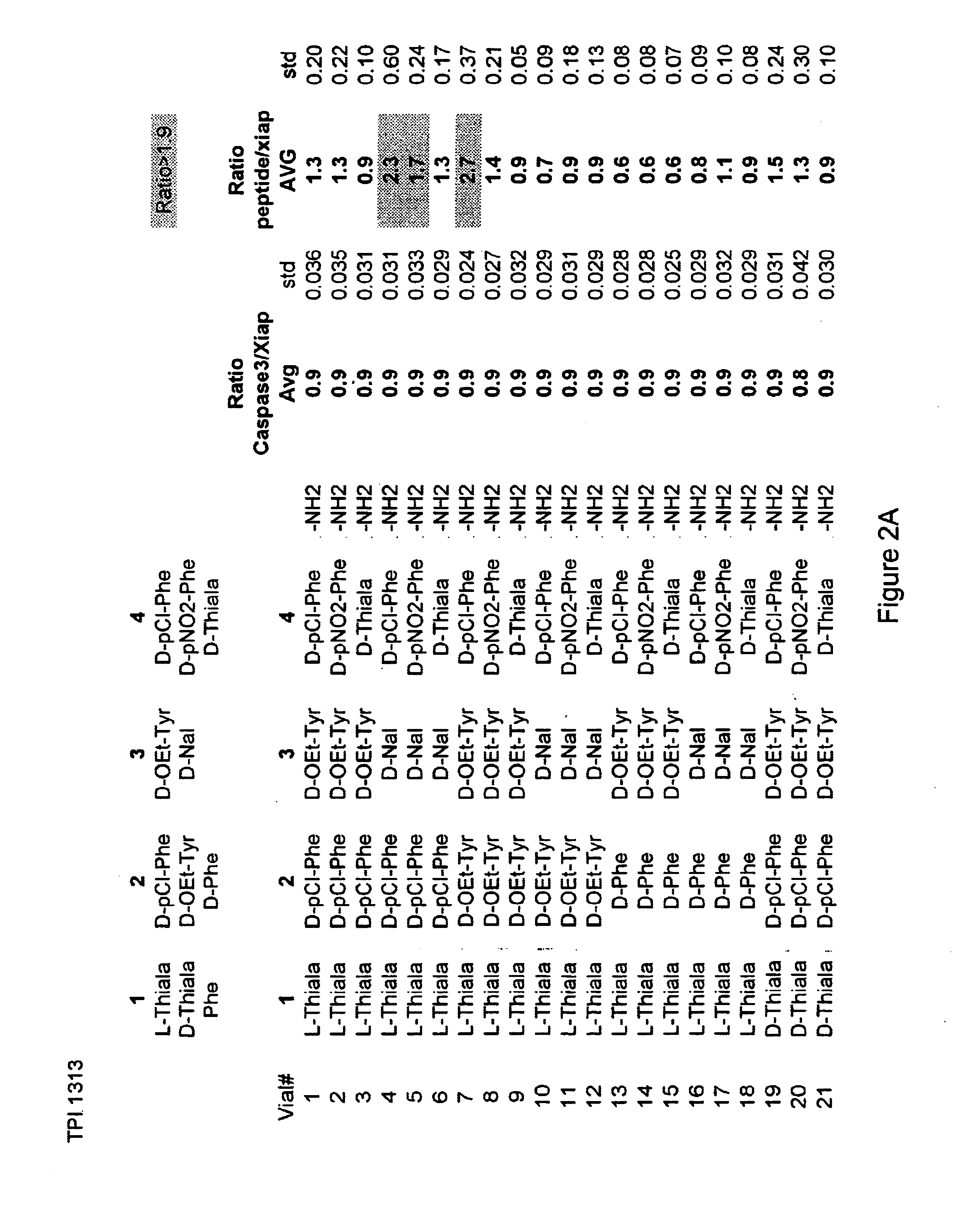
The invention provides isolated agents having a core peptide selected from the group consisting of Core peptides 5 through 39 and 42 through 55, wherein the agent derepresses an IAP-inhibited caspase. Also provided is an isolated agent having a core structure selected from any of the structures shown in FIGS. 5, 9, 10, 14B, 21-24 and 48, a core structure selected from the group of TPI 759, TPI 882, TPI 914 or TPI 927; and a core structure from a library selected from TPI 1391, TPI 1349, TPI 1396, TPI 1509, TPI 1540, TPI 1400, TPI 792, TPI 1332, TPI 1567, TPI 1576 and TPI 1577, wherein the agent derepresses an IAP-inhibited caspase. The invention further provides a method of derepressing an IAP-inhibited caspase.
Owner:THE BURNHAM INST
Methods and compositions for derepression of IAP-inhibited caspase
InactiveUS6911426B2Promote apoptosisReduce severityHydrolasesPeptide/protein ingredientsDerepressionApoptosis
The invention provides isolated agents having a core peptide selected from the group consisting of Core peptides 5 through 39 and 42 through 55, wherein the agent derepresses an IAP-inhibited caspase. Also provided is an isolated agent having a core structure selected from any of the structures shown in FIGS. 5, 9, 10, 14B, and 21-24 wherein said agent derepresses an IAP-inhibited caspase. The invention further provides a method of derepressing an IAP-inhibited caspase. The method consists of contacting an IAP-inhibited caspase with an effective amount of an agent to derepress an IAP-inhibited caspase, the agent having a core motif selected from the group consisting of a core peptide having a sequence set forth in any of Core peptides 4 through 39 and 42 through 55, and a core structure selected from the group consisting of TPI759, TPI882, TPI914 or TPI927. The methods of the invention also can be used for promoting apoptosis in a cell and for reducing the severity of a pathology characterized by reduced levels of apoptosis. Methods for identifying agents that derepress an IAP-inhibited caspase are also provided.
Owner:TORREY PINES INST FOR MOLECULAR STUDIES +1
Systemic discovery, maturation and extension of peptide binders to proteins
Owner:ROCHE SEQUENCING SOLUTIONS INC
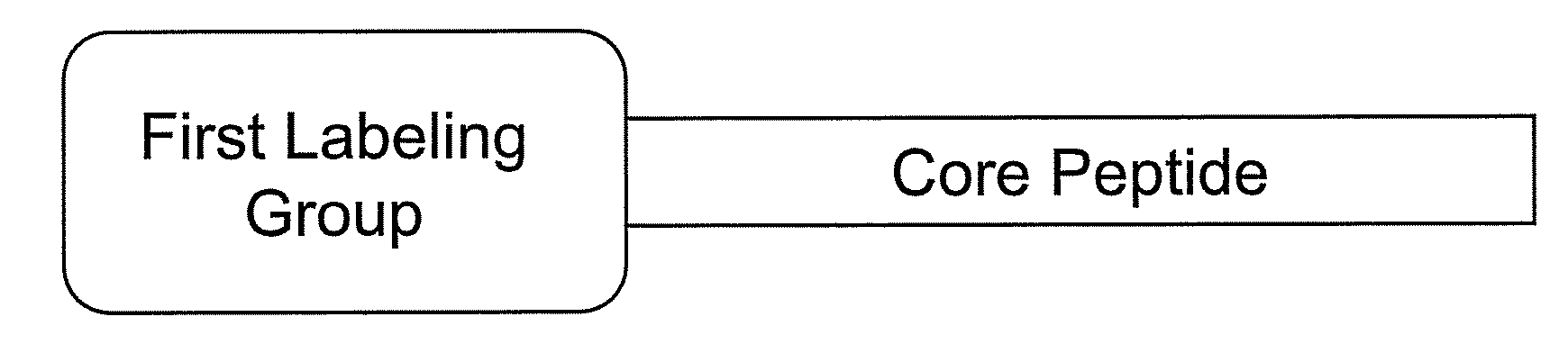
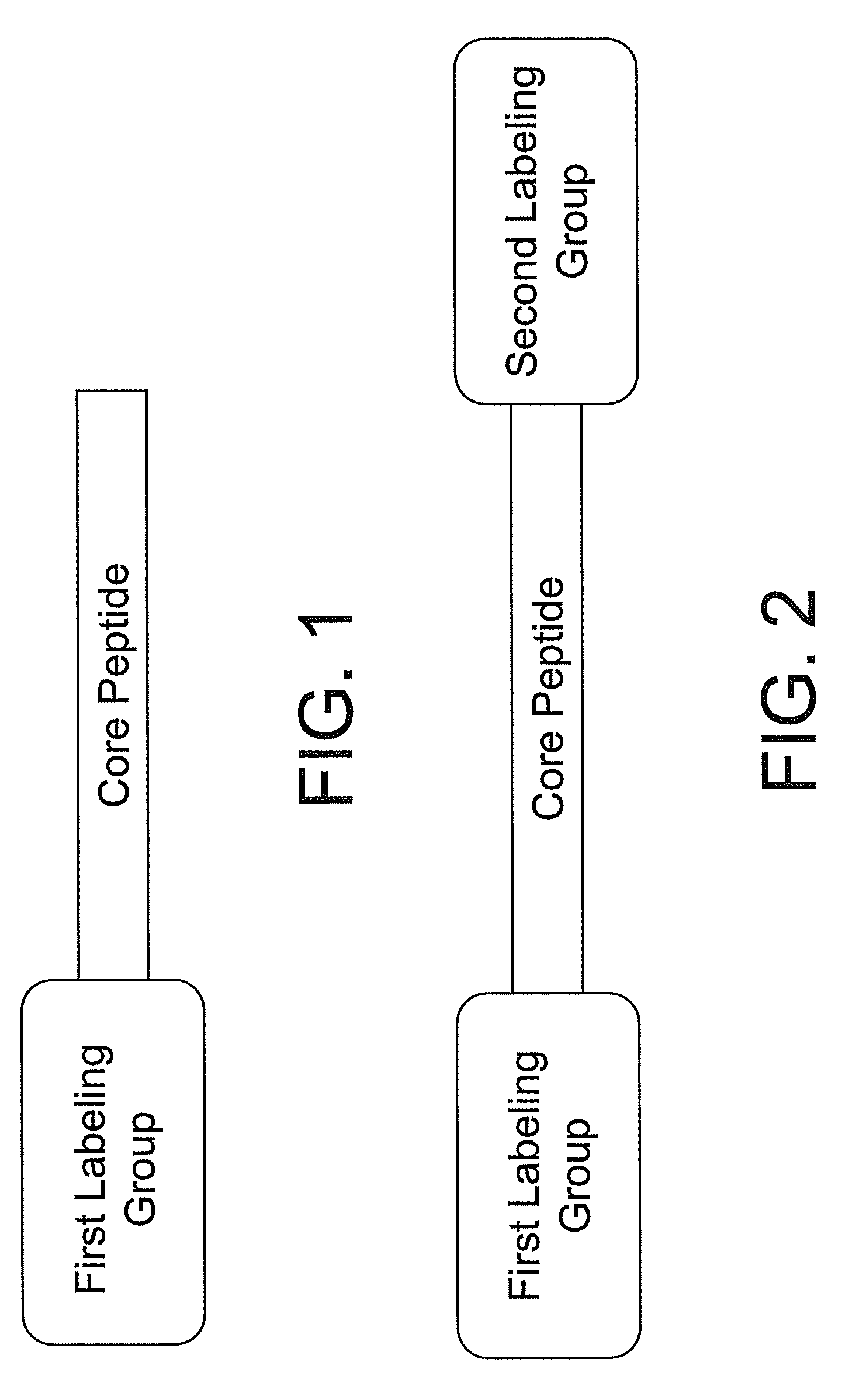
Smart contrast agent and method for detecting transition metal ions and treating related disorders
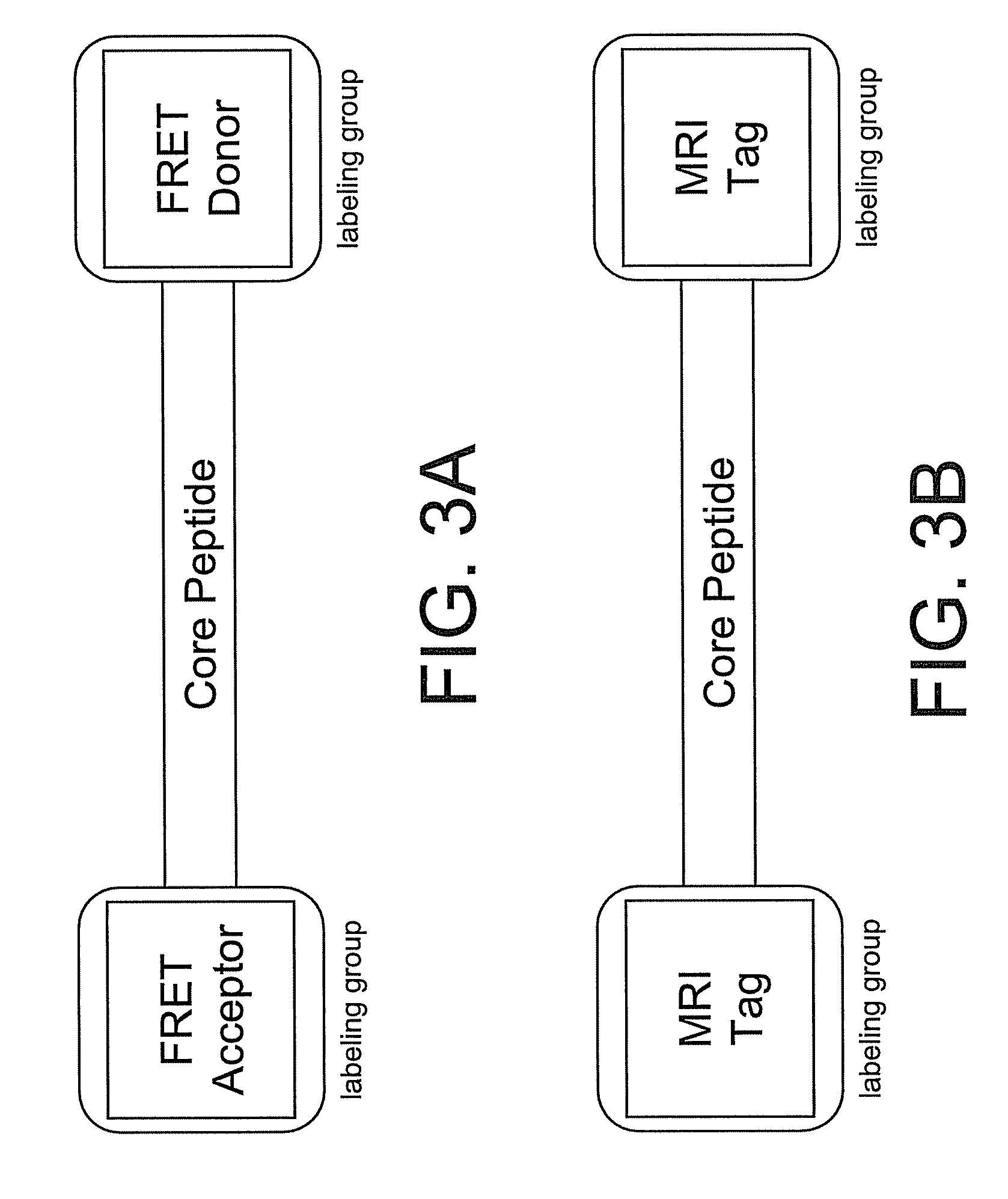
InactiveUS20100227794A1Reduce negative impactNervous disorderIn-vivo radioactive preparationsRelated disorderCore peptide
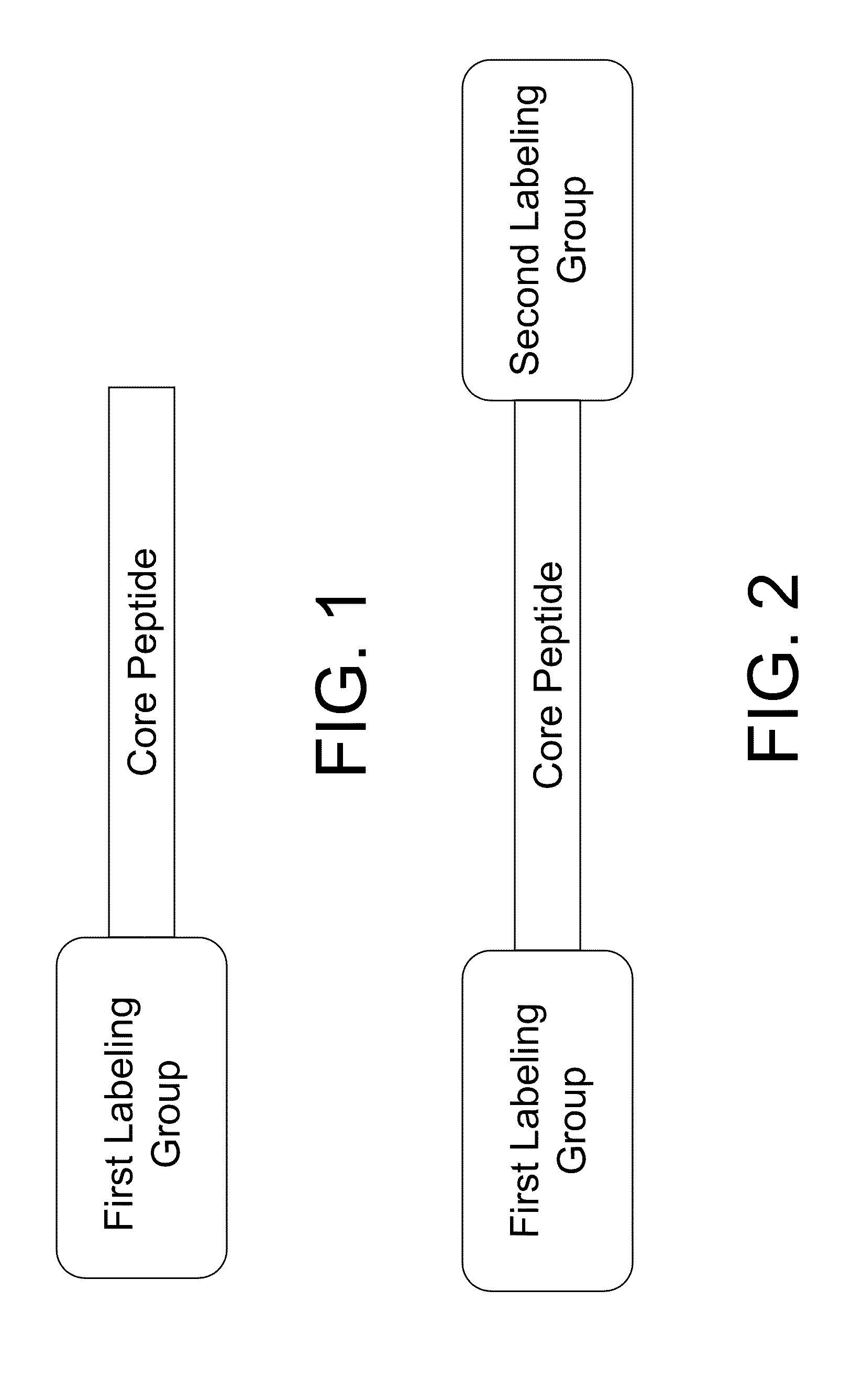
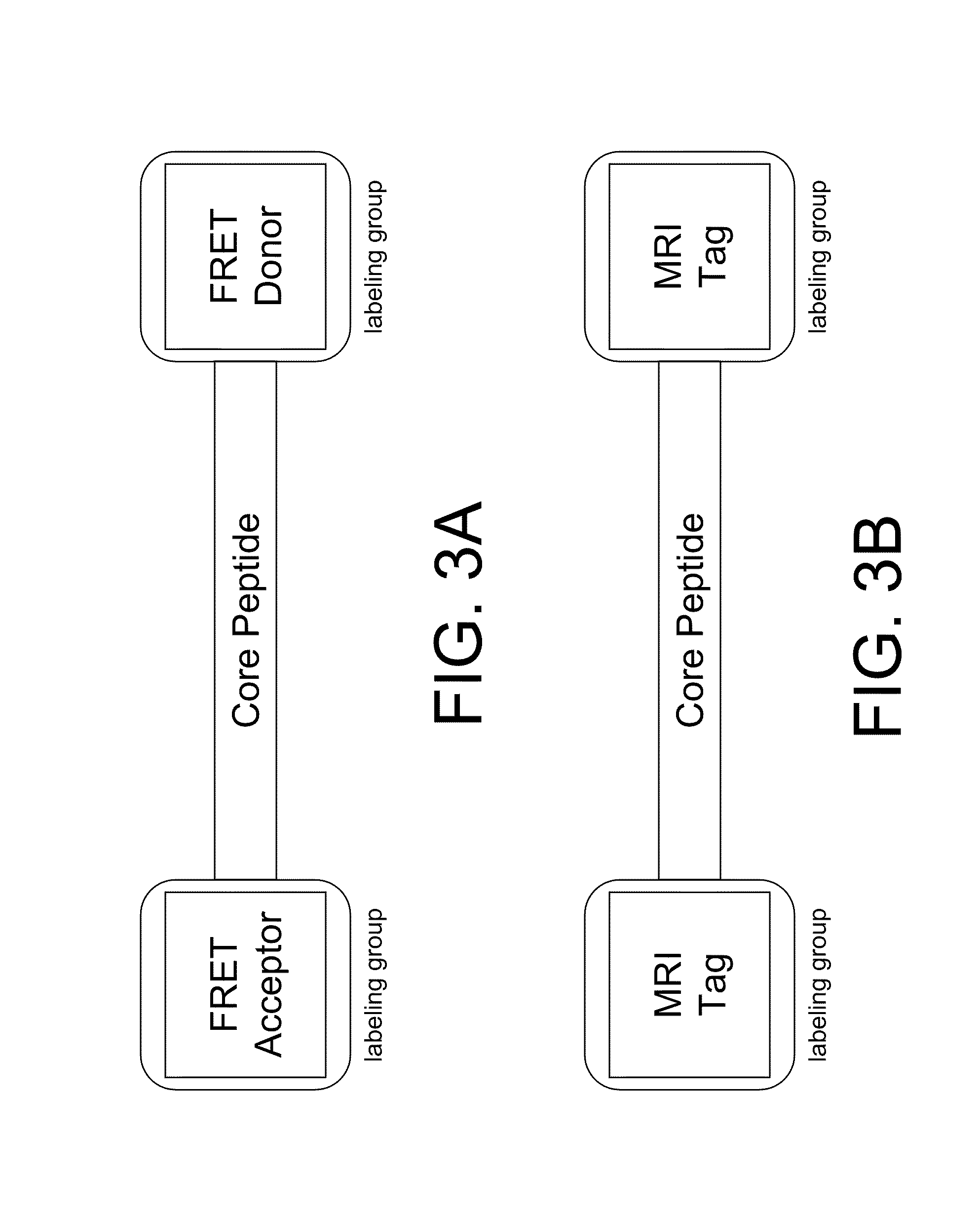
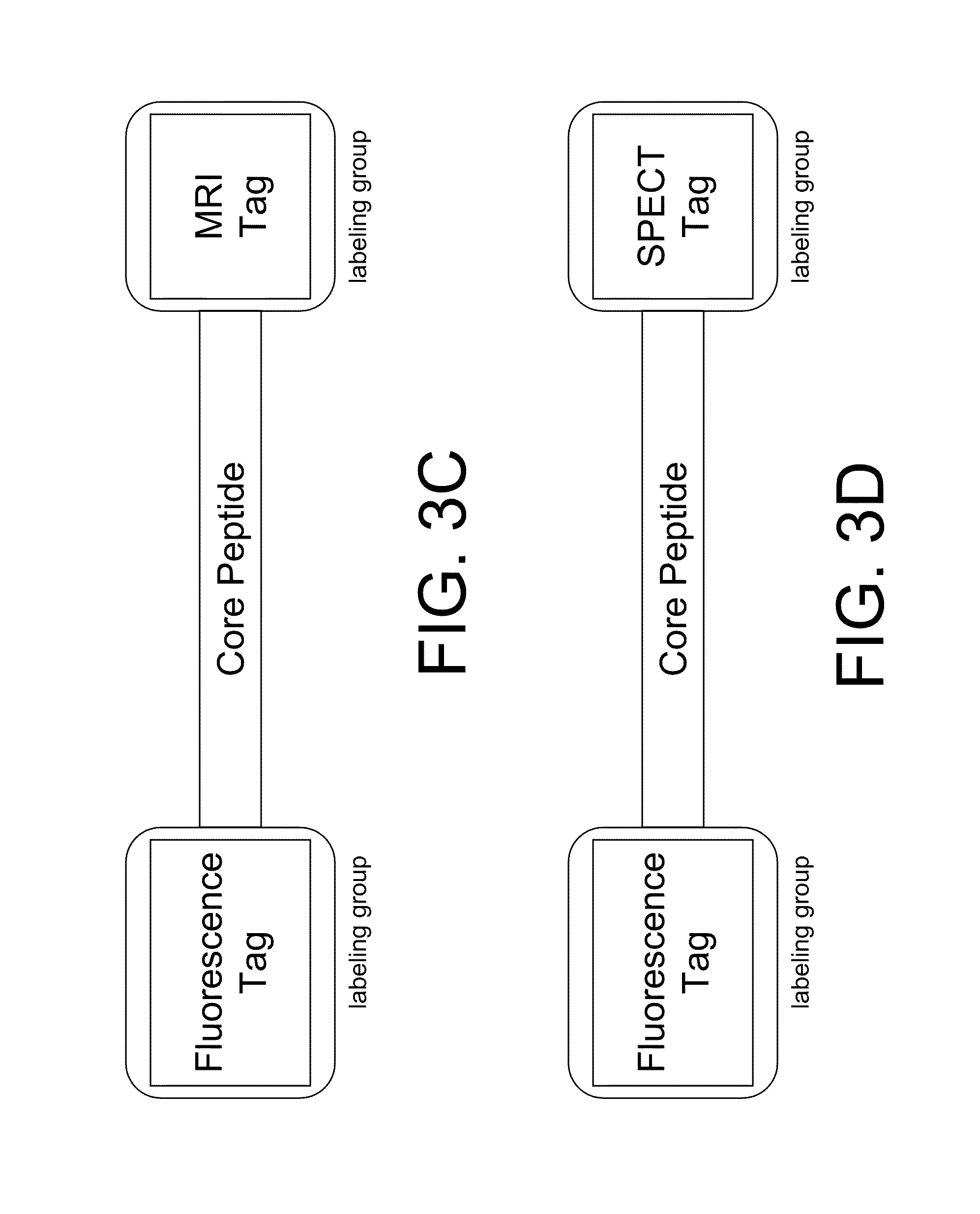
The present disclosure provides smart contrast agents for transition metals and a method of using the same. The smart contrast agents include a core peptide and a first labeling group attached to a first end of the core peptide. The smart contrast agents can also include a second labeling group attached to a second end of the core peptide. The core peptide can bind to transition metals, and can be homologous to a fragment selected from the extended octarepeat region of a prion protein.
Owner:I S T CORP
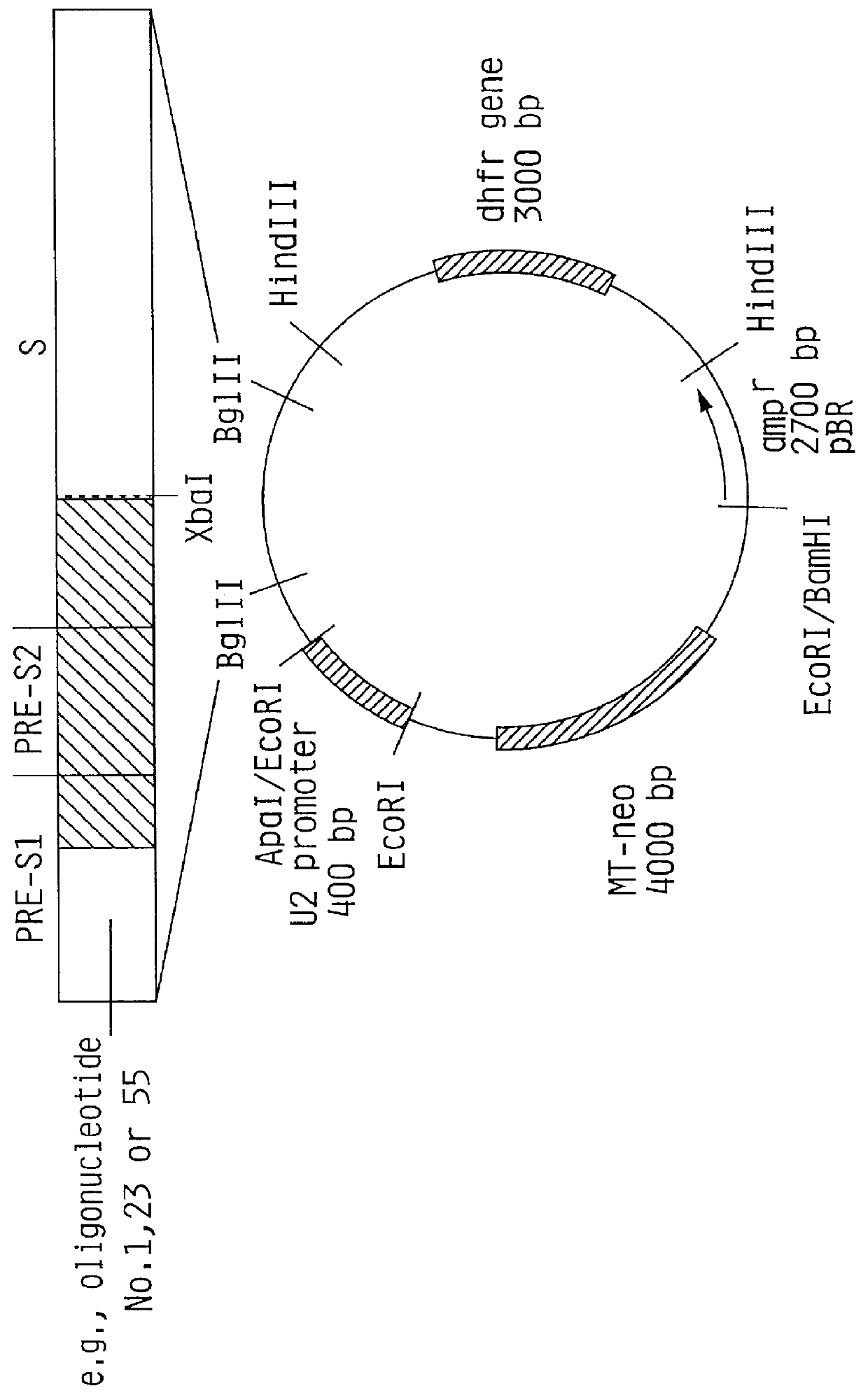
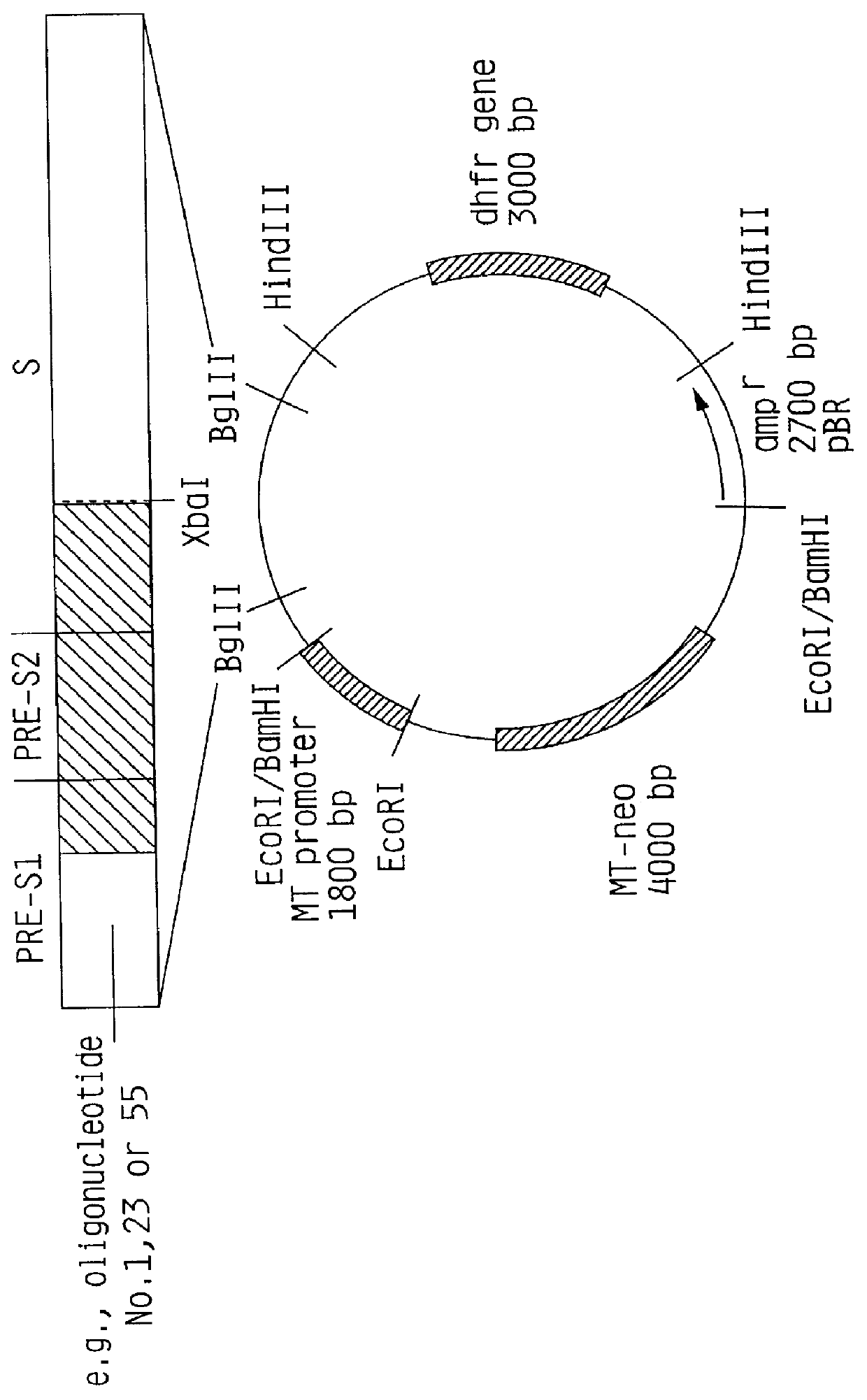
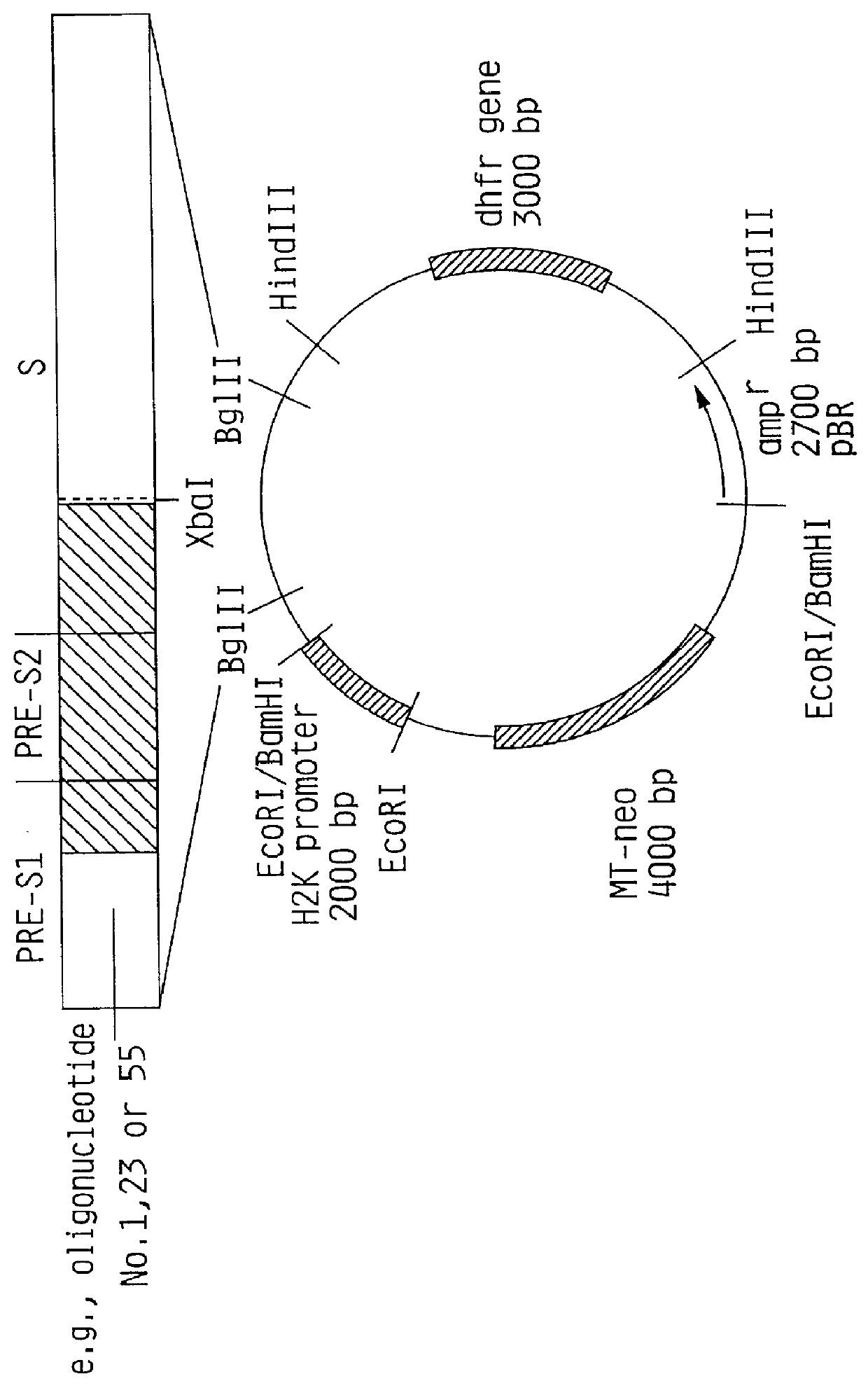
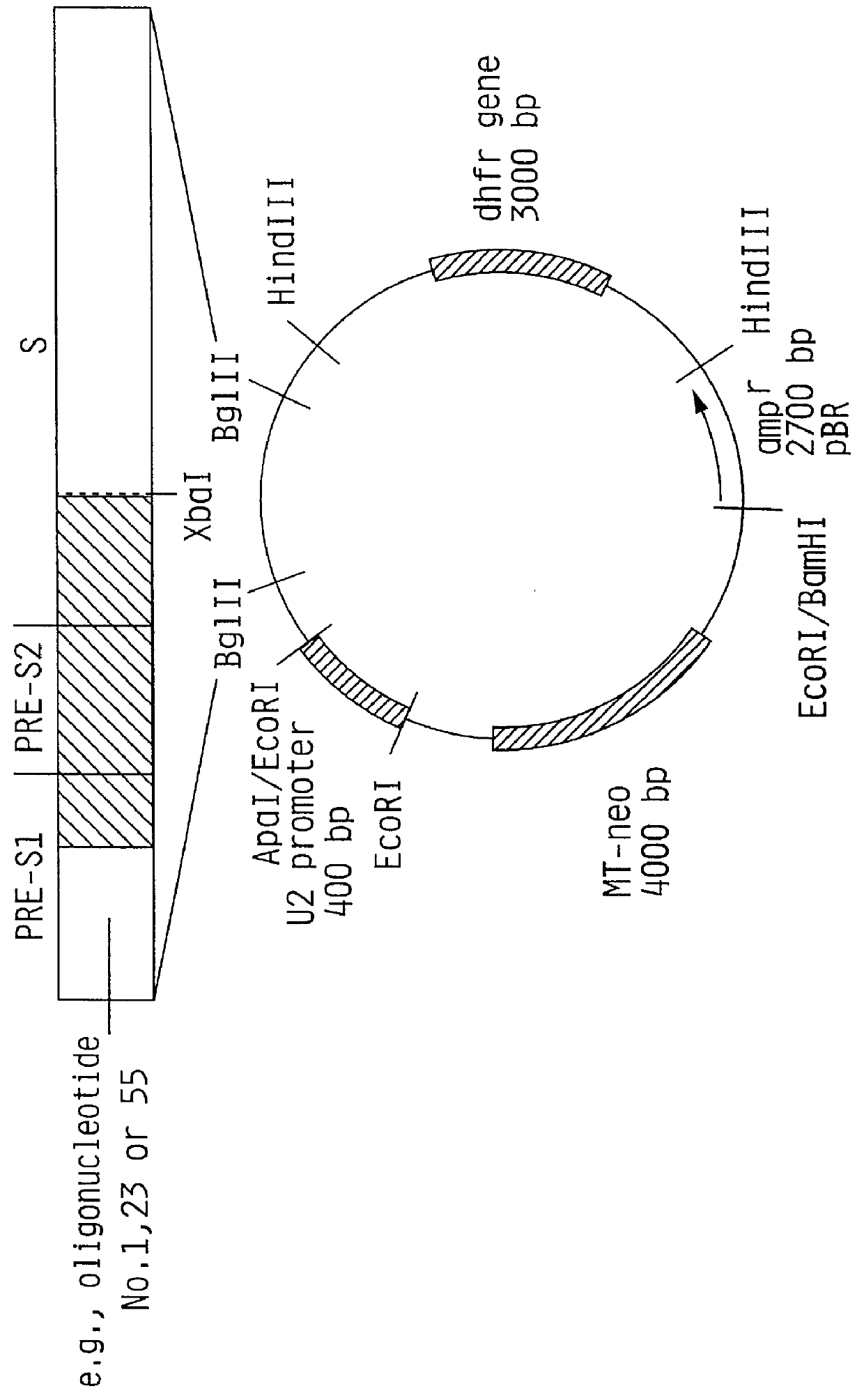
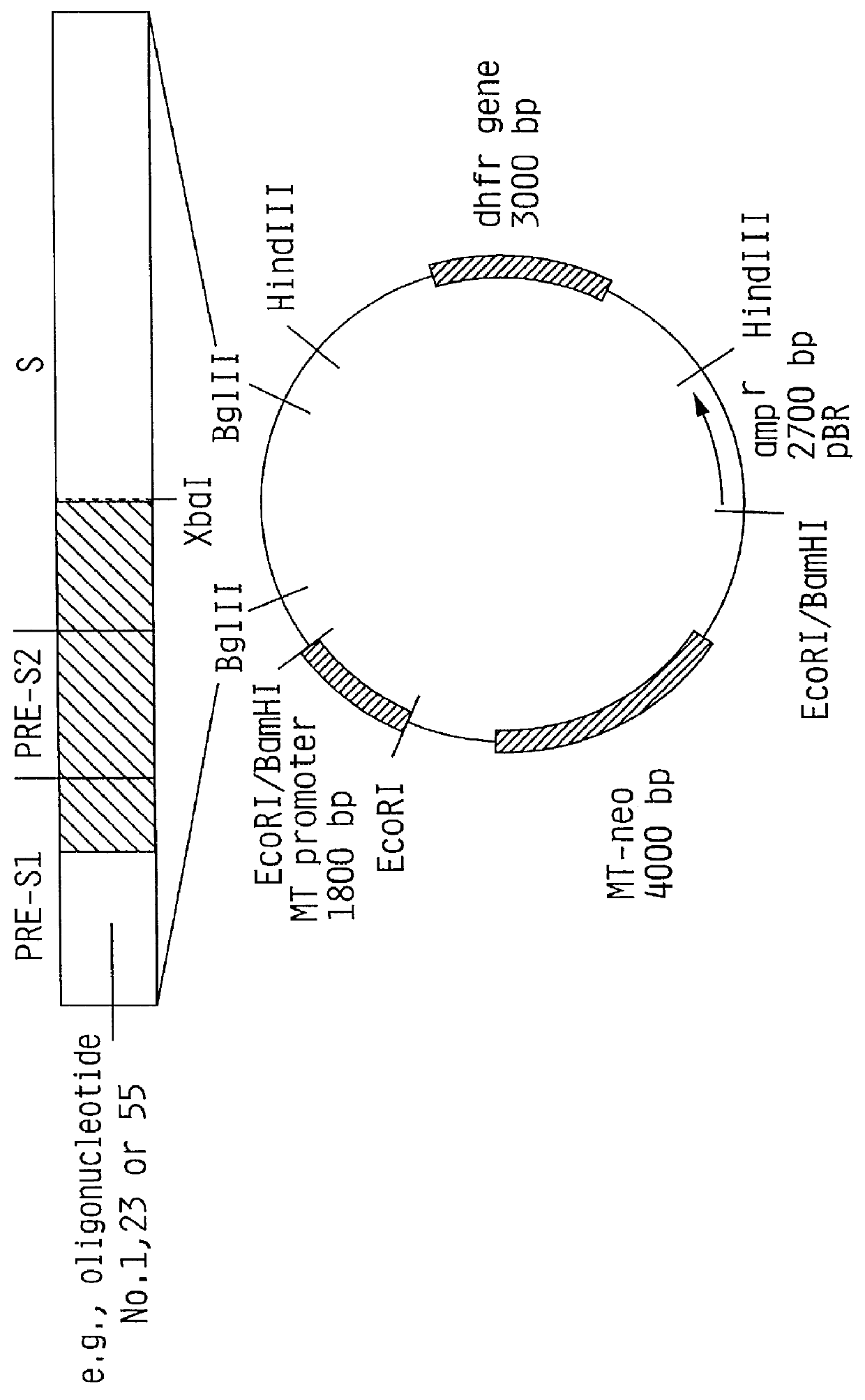
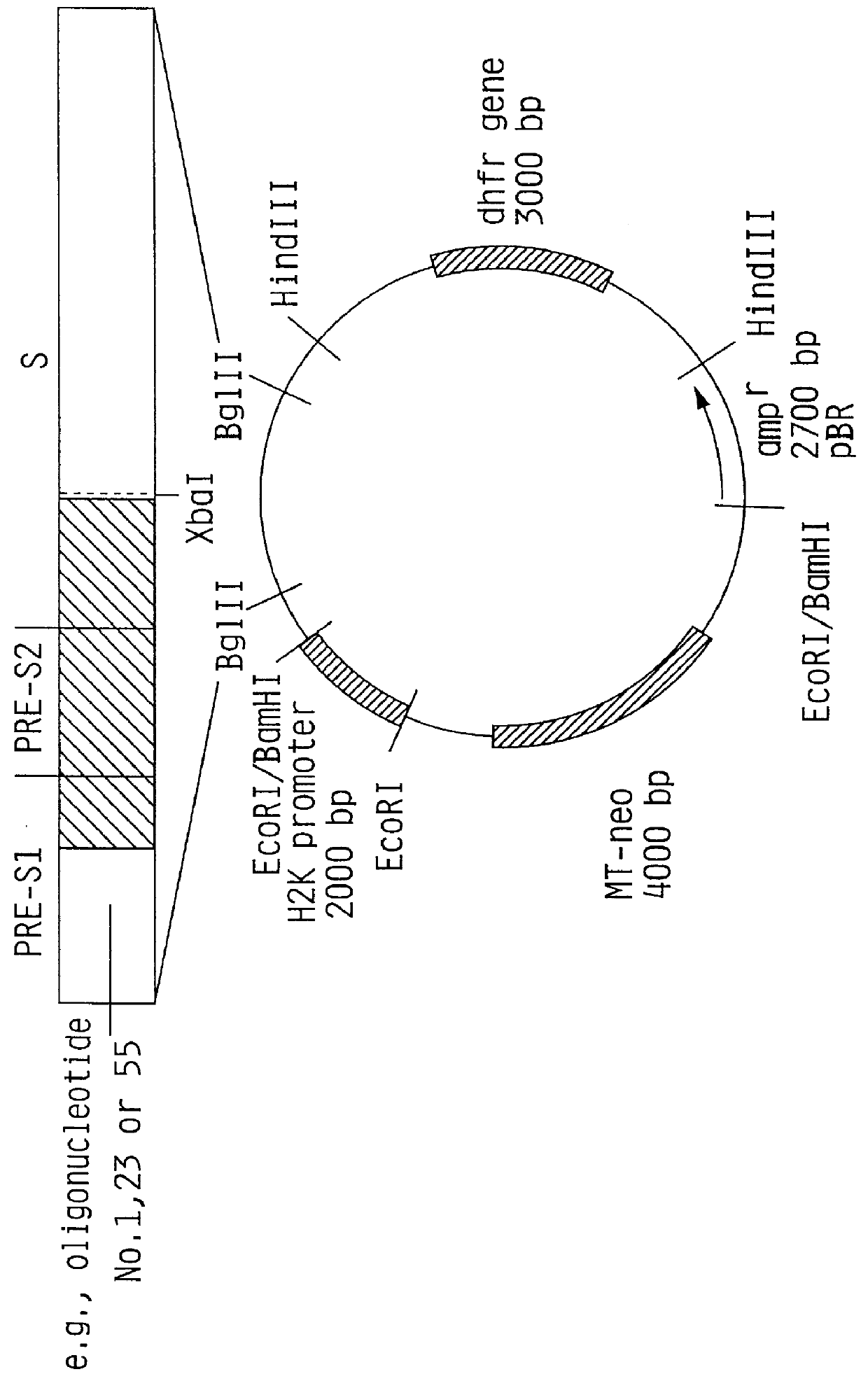
Hepatitis B surface antigen vaccine
InactiveUS6022543AGood antigenicityStimulate immune responseSsRNA viruses positive-senseBacteriaEukaryotic plasmidsHepatitis B virus
HBV surface antigen particles, prepared by recombinant DNA technology are described, said particles being composed of epitopes from the group of surface peptides and / or core peptide of non-A, non-B hepatitis virus, hepatitis virus A and / or hepatitis virus B. Respective particles are especially characterized by a composition of different epitopes selected from pre-S and S peptides. There are also described DNA-sequences, plasmids and cell lines coding for respective HBV surface antigen particles as well as a new vaccine containing the same.
Owner:MEDEVA HLDG
Hepatitis B surface antigen vaccine
InactiveUS6072049AGood antigenicityStimulate immune responseVirusesBacteriaEukaryotic plasmidsHepatitis B virus
HBV surface antigen particles, prepared by recombinant DNA technology are described, said particles being composed of epitopes from the group of surface peptides and / or core peptide of non-A, non-B hepatitis virus, hepatitis virus A and / or hepatitis virus B. Respective particles are especially characterized by a composition of different epitopes selected from pre-S and S peptides. There are also described DNA-sequences, plasmids and cell lines coding for respective HBV surface antigen particles as well as a new vaccine containing the same.
Owner:MEDEVA HLDG
Smart contrast agent and detection method for detecting transition metal ions
The present disclosure provides smart contrast agents for transition metals and a method of using the same. The smart contrast agents include a core peptide and a first labeling group attached to a first end of the core peptide. The smart contrast agents can also include a second labeling group attached to a second end of the core peptide. The core peptide can bind to transition metals, and can be homologous to a fragment selected from the extended octarepeat region of a prion protein.
Owner:I S T CORP
Methods and compositions for derepression of IAP-inhibited caspase
InactiveUS20050119176A1Promote apoptosisReduce severityPeptide/protein ingredientsAntipyreticDerepressionCaspase
The invention provides isolated agents having a core peptide selected from the group consisting of Core peptides 5 through 39 and 42 through 55, wherein the agent derepresses an IAP-inhibited caspase. Also provided is an isolated agent having a core structure selected from any of the structures shown in FIGS. 5, 9, 10, 14B, and 21-24, a core structure selected from the group of TPI 759, TPI 882, TPI 914 or TPI 927; and a core structure from a library selected from TPI 1391, TPI 1349, TPI 1396, TPI 1509, TPI 1540, TPI 1400, TPI 792 and TPI 1332, wherein the agent derepresses an IAP-inhibited caspase. The invention further provides a method of derepressing an IAP-inhibited caspase.
Owner:BURNHAM INST THE +1
Nanoparticles for controlling bleeding and drug delivery
InactiveUS20140242180A1Shorten bleeding timeEffective amountPowder deliveryTetrapeptide ingredientsNanoparticleWater soluble
A temperature stable nanoparticle is provided comprising a core, a water soluble polymer and a peptide, the water soluble polymer attached to the core at a first terminus of the water soluble polymer, the peptide attached to a second terminus of the water soluble polymer, the peptide comprising an RGD amino acid sequence, the water soluble polymer of having sufficient length to allow binding of the peptide to glycoprotein lib / Ilia (GPIIb / llla). In one aspect, the nanoparticle has a melting temperature over 35° C. In various aspects, the nanoparticle has a spheroid shape and a diameter of less than 1 micron.
Owner:CASE WESTERN RESERVE UNIV
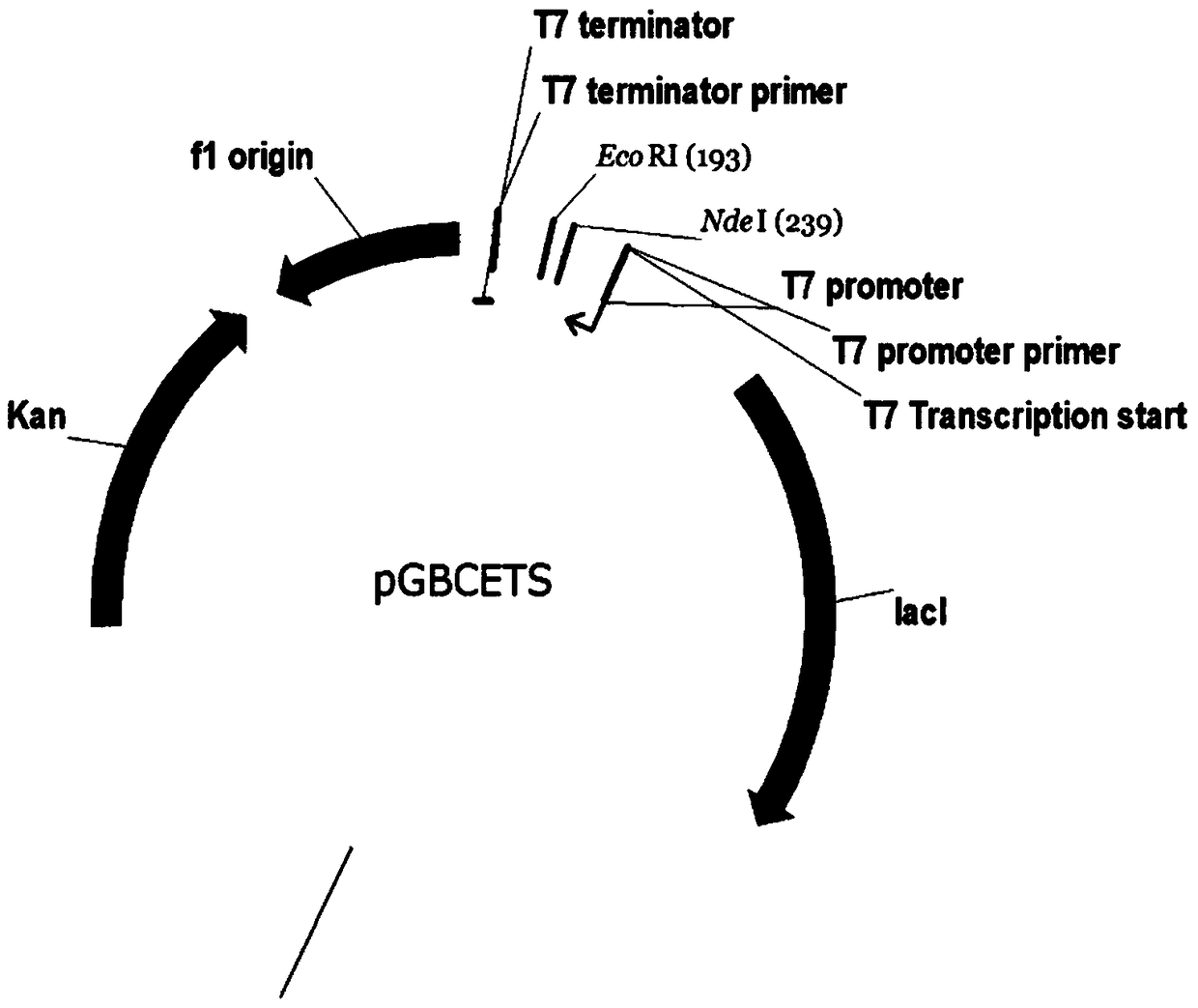
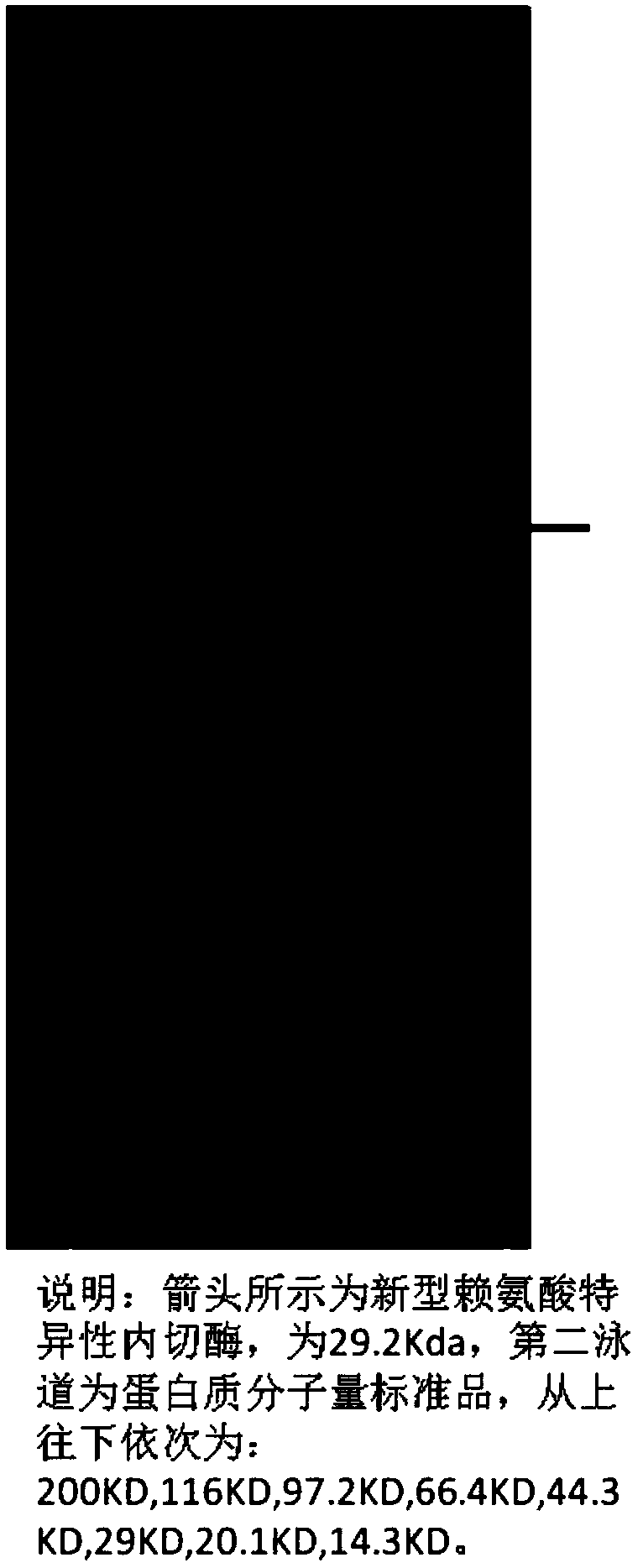
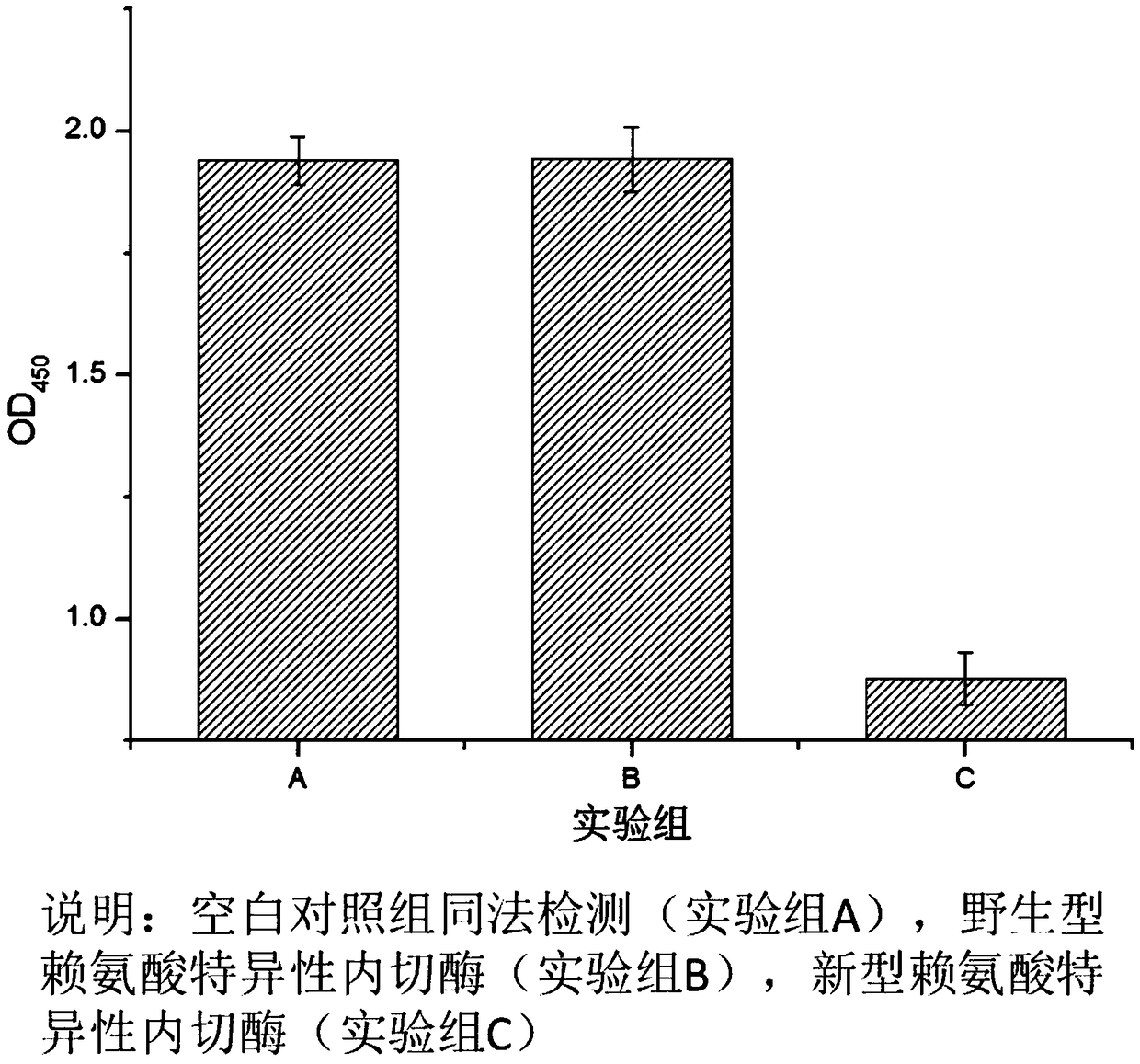
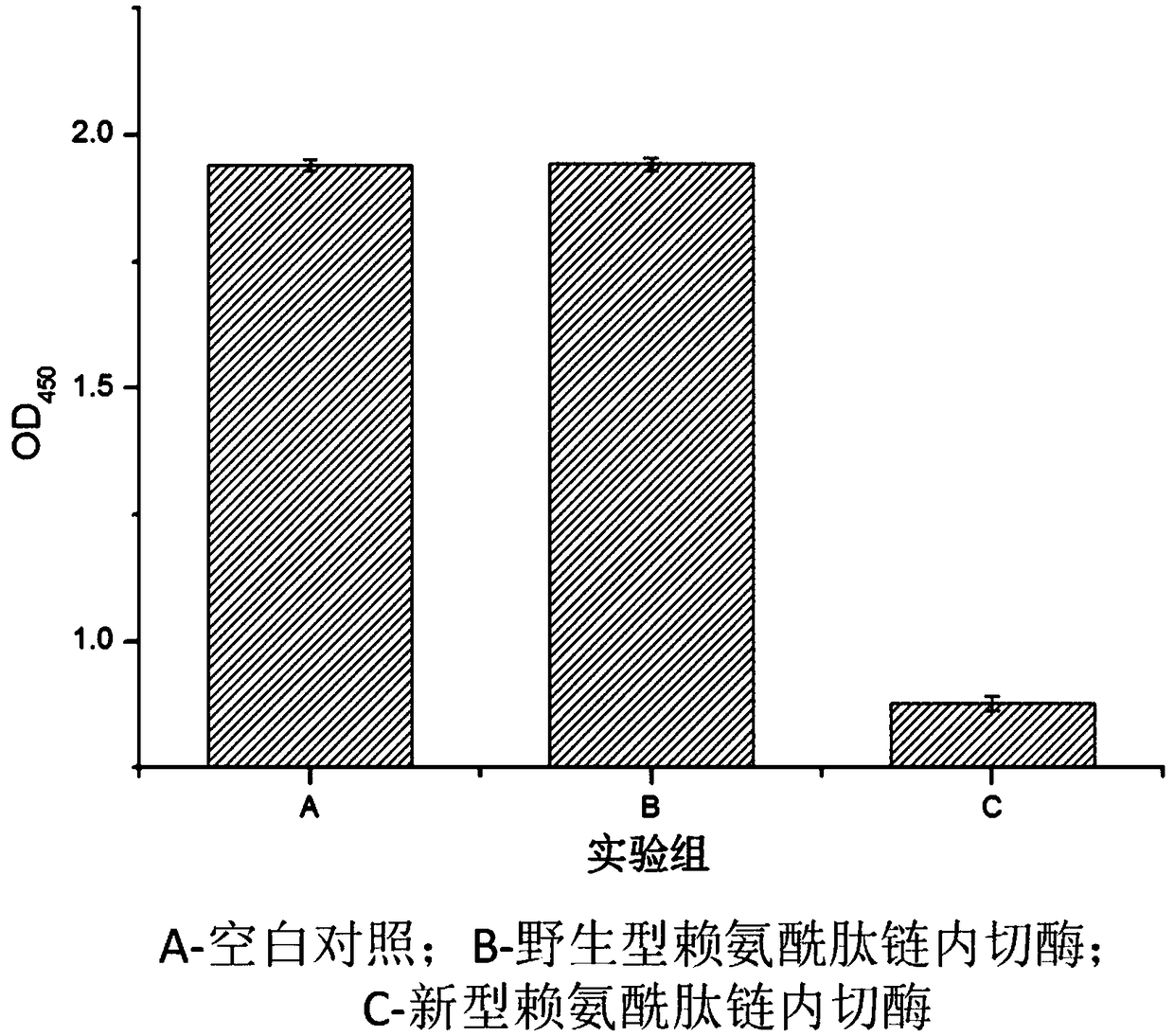
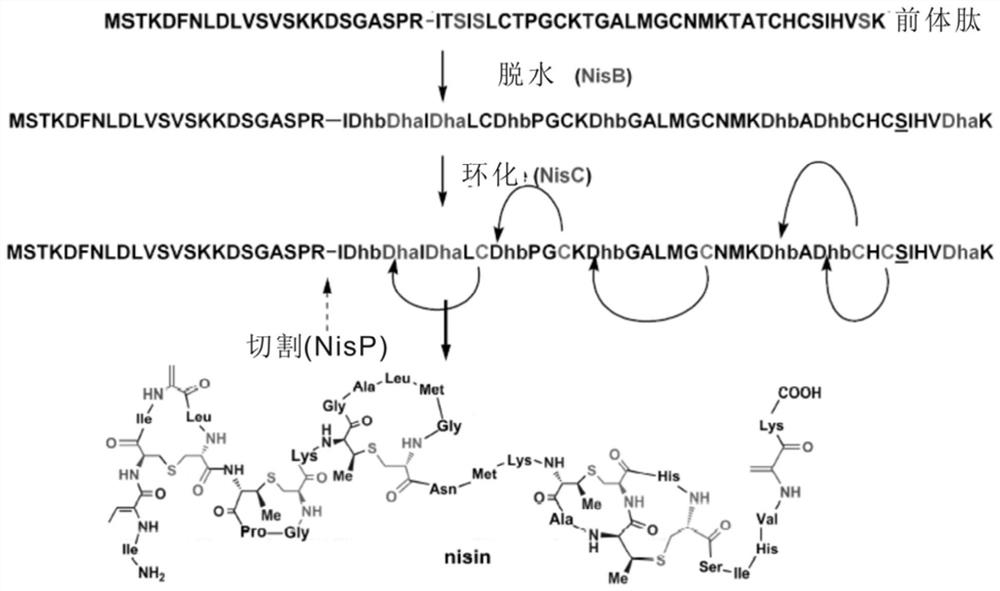
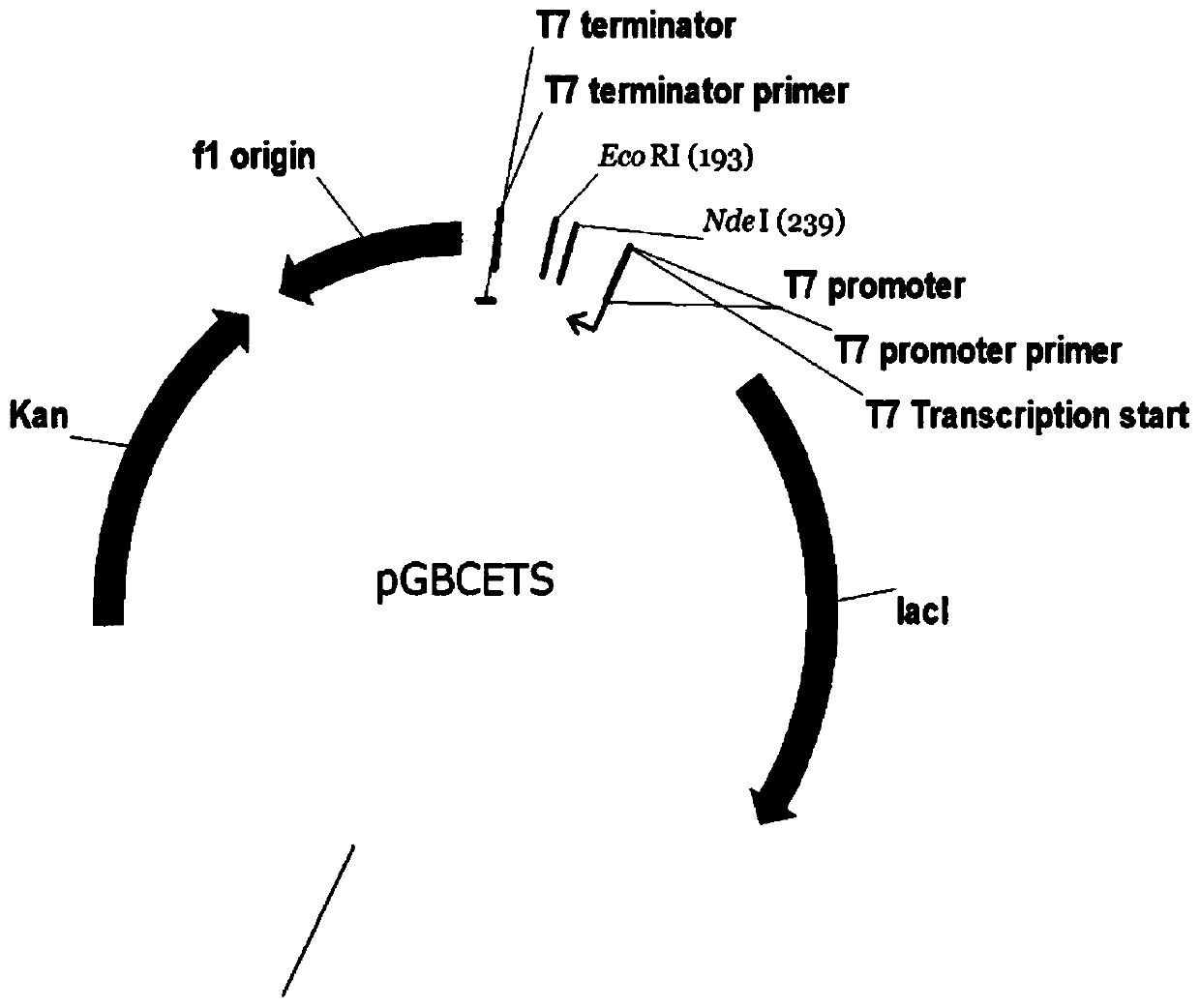
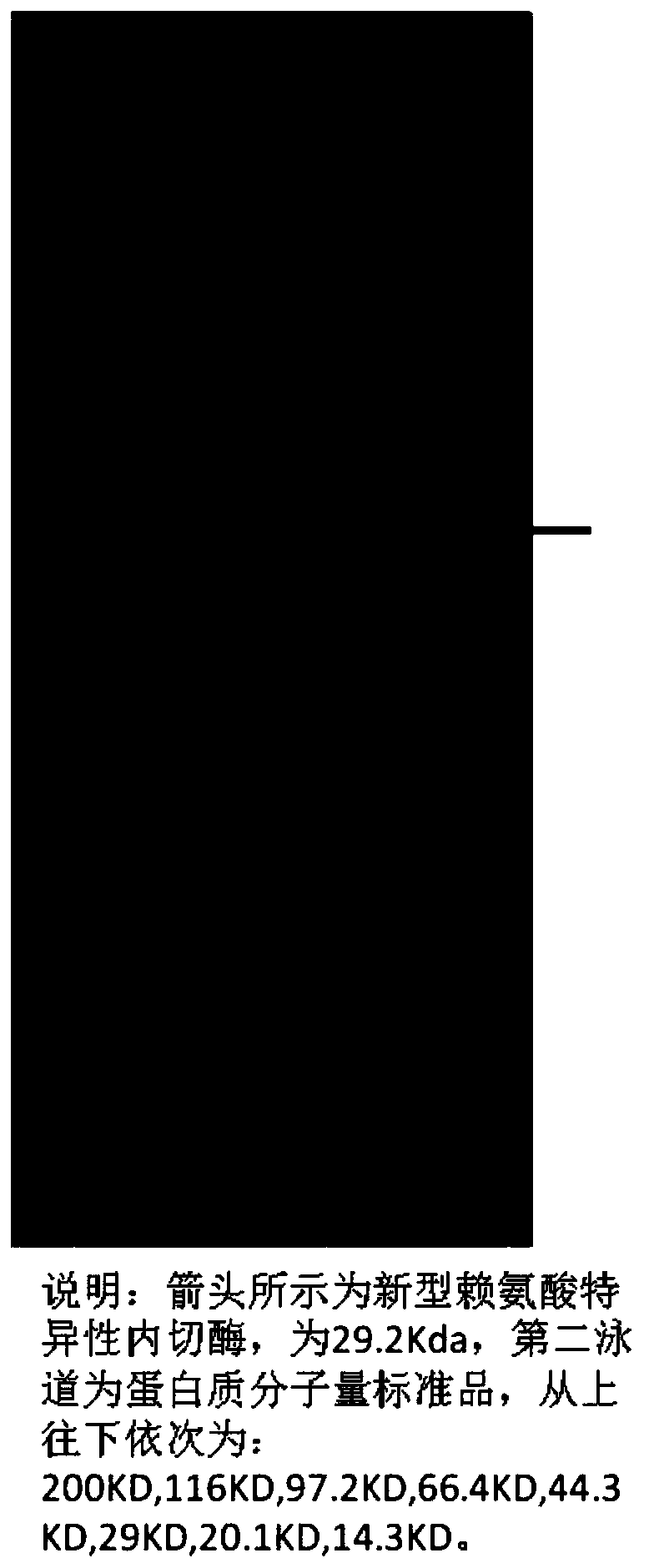
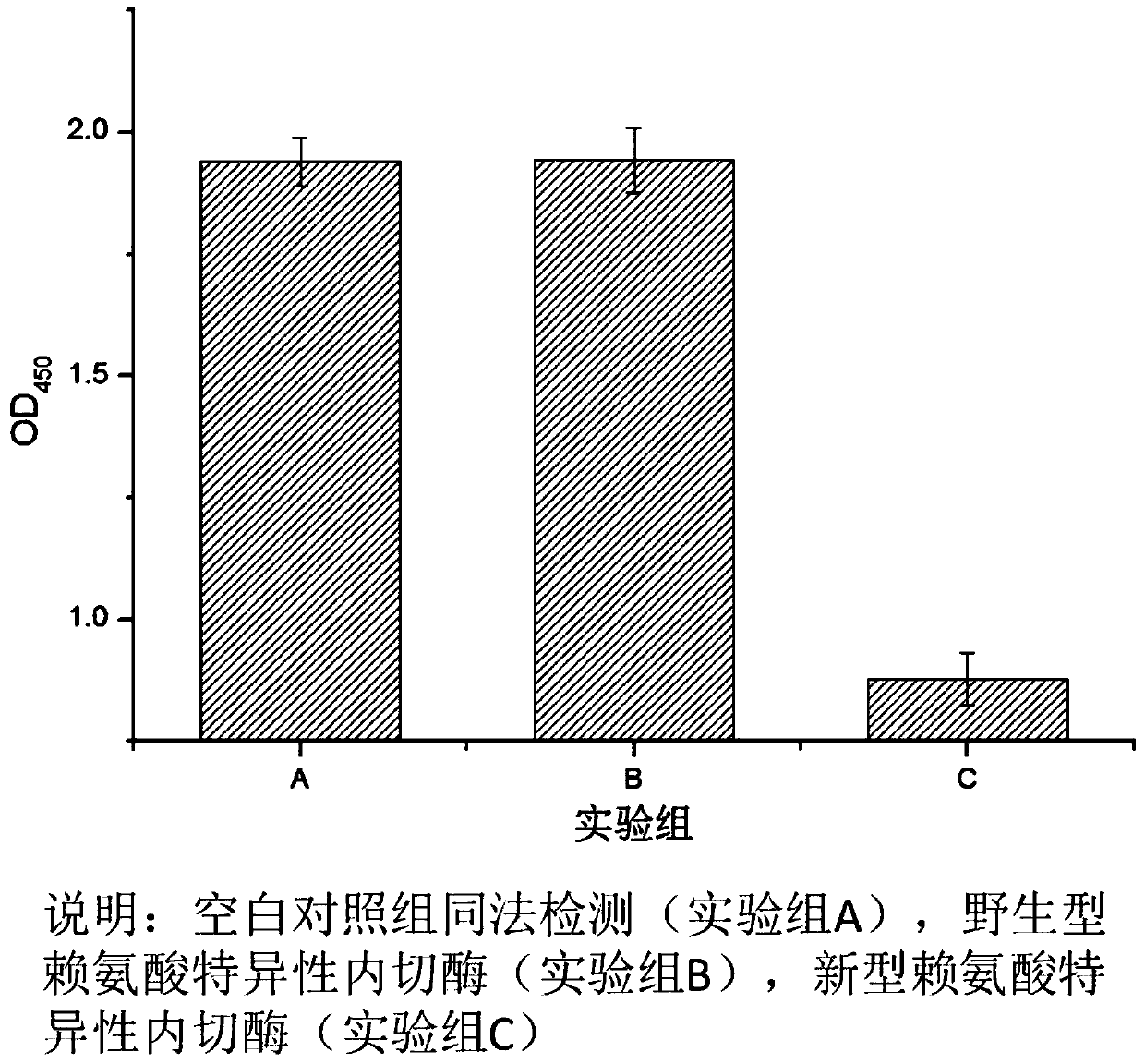
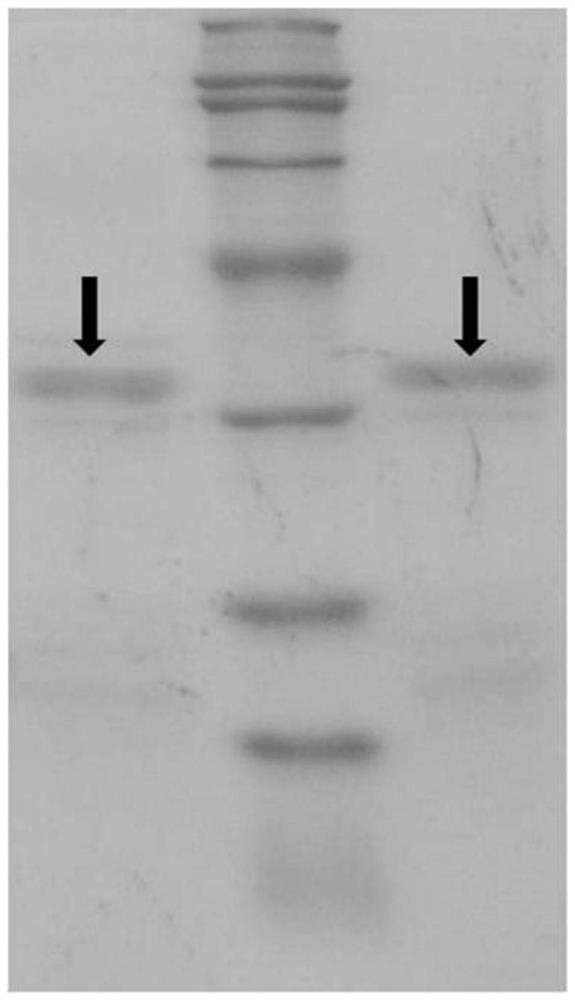
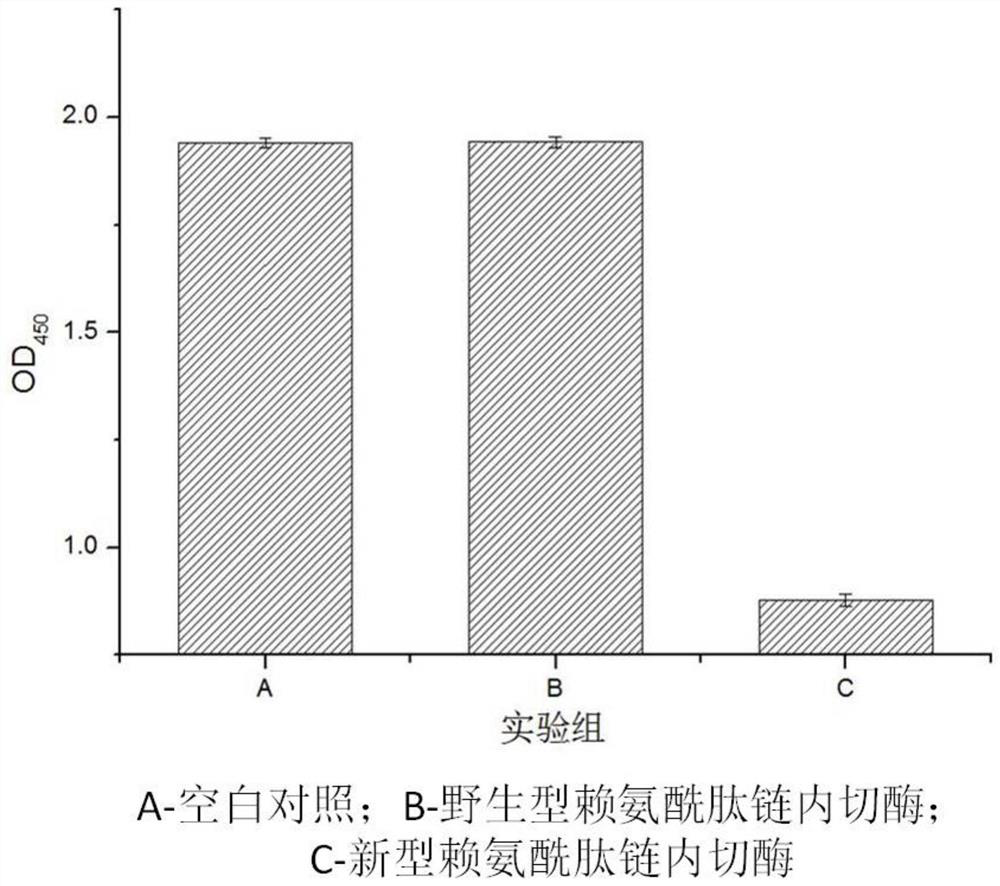
Novel lysyl endopeptidase and preparation method thereof
ActiveCN109486800AHas whole enzyme activityReserved functionVector-based foreign material introductionPeptidasesArginineIon exchange
The invention discloses novel lysyl endopeptidase. A suitable flexible linker peptide is connected to an enzyme core peptide, a histidine label and an arginine tail end, while the original enzymatic activity is maintained, nanogram-level residual enzyme in a sample is detected by an ELISA method, and furthermore, novel lysyl endopeptidase sterling is obtained by purification of an ion exchange layer. The invention further provides a preparation method of the lysyl endopeptidase, after 1L of fermentation broth is purified, the recycling amount of 107 mg of the novel lysyl endopeptidase sterlingcan be achieved, and the novel lysyl endopeptidase has industrial potential.
Owner:ZHUHAI JINBAIKANG BIOLOGICAL TECH CO LTD
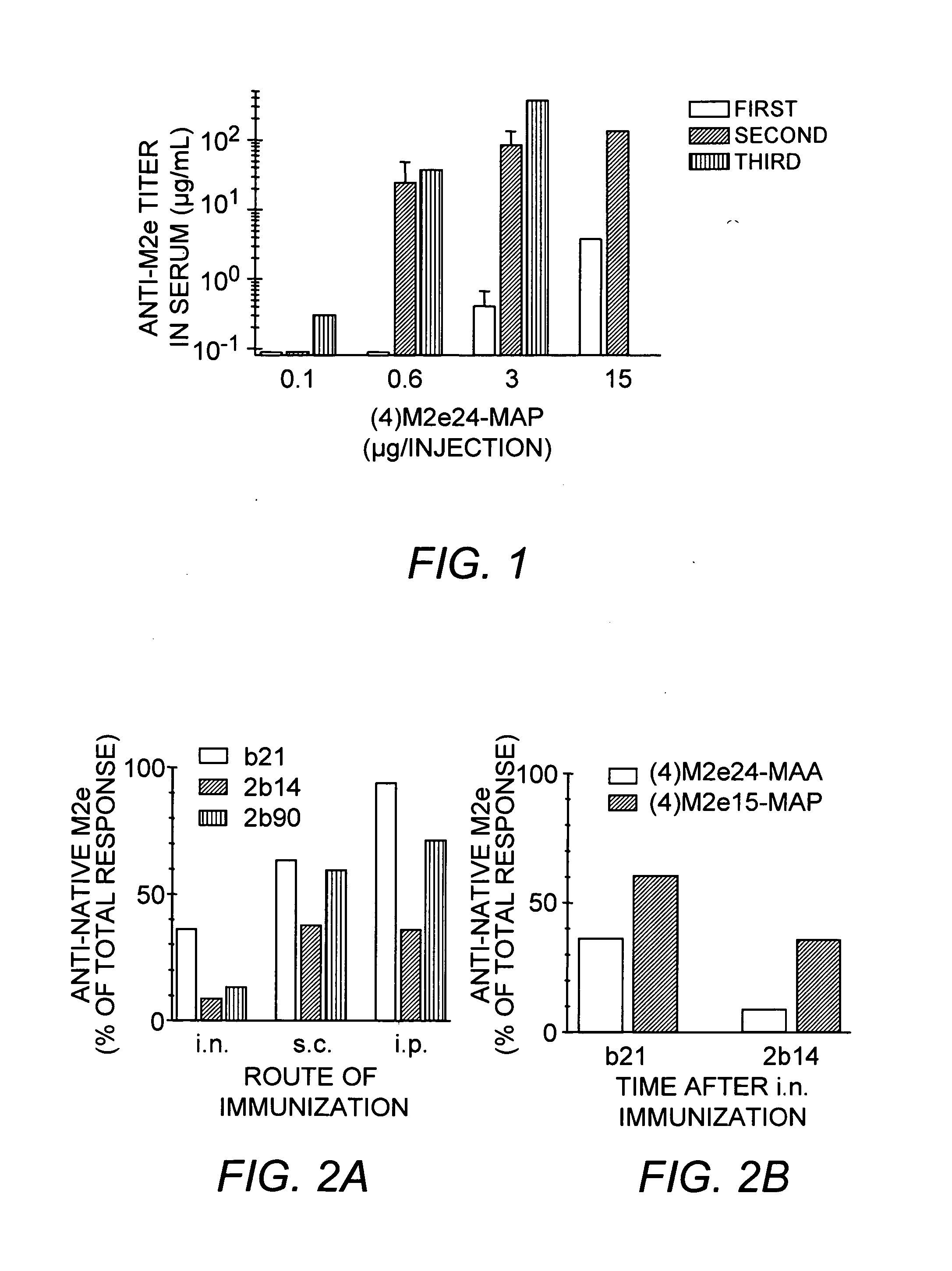
Multiple antigenic agents and methods for using the same
The present invention provides multiple antigenic agents compositions and the use thereof to prevent or treat viral infections. The multiple antigenic agents of the invention contain at least one of a B cell determinant, a T cell determinant, or a targeting molecule attached to a core peptide composed of Lys-Gly repeats.
Owner:WISTAR INSTITUTE
Novel lysyl endopeptidase and preparation method thereof
ActiveCN109439643AHas whole enzyme activityReserved functionFermentationVector-based foreign material introductionWild typePolyhistidine-tag
The invention discloses novel lysyl endopeptidase. An enzyme core peptide is connected with histidine tags through an appropriate flexible linker peptide, so that nanogram residual enzyme in a samplecan be detected by an ELISA (enzyme-linked immunosorbent assay) method; and total activity of wild lysyl endopeptidase is ensured. At the same time, the invention further provides a preparation methodof the lysyl endopeptidase. Compared with a gene engineering modification and fermentation technology, the preparation method has the benefit of high yield; a recovery amount of 857mg novel lysyl endopeptidase can be obtained after purification of 1L fermentation liquid; and the method has an industrial potential.
Owner:ZHUHAI JINBAIKANG BIOLOGICAL TECH CO LTD
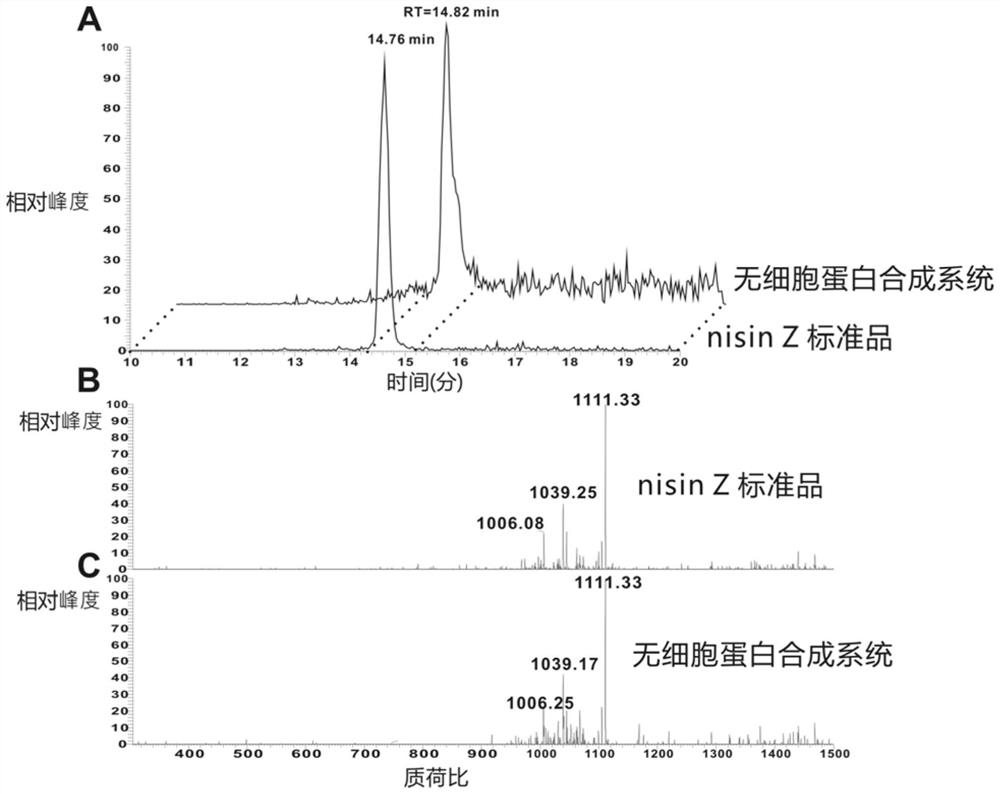
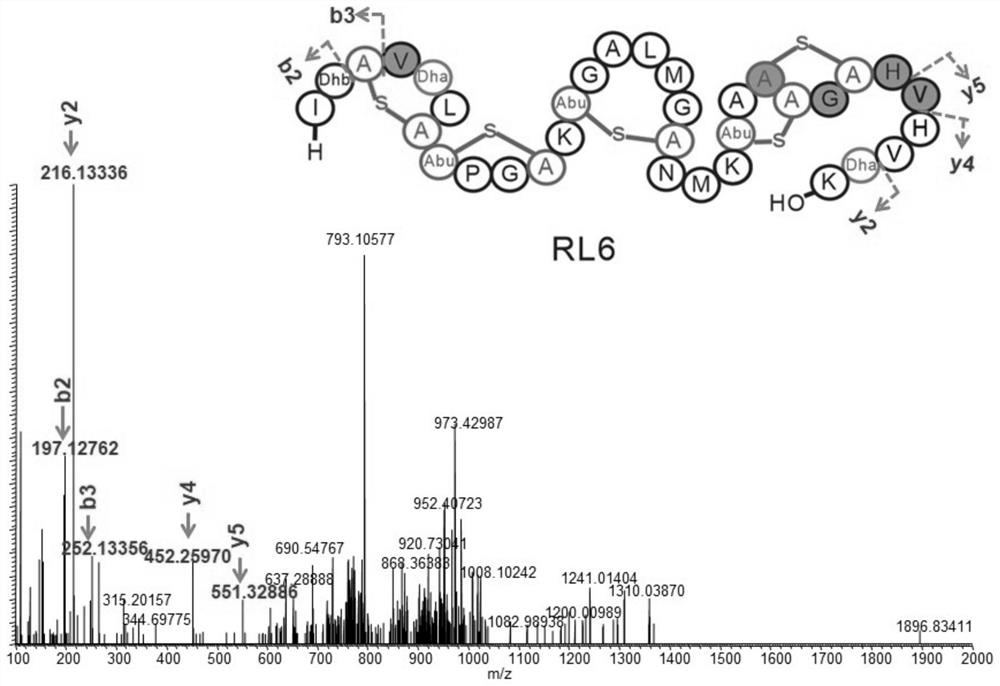
Screening method of wool thiopeptide, cell-free protein synthesis system and wool thiopeptide
The invention relates to the field of bioengineering, in particular to a screening method of wool thiopeptide, a cell-free protein synthesis system and the wool thiopeptide. The screening method comprises the following steps of (1) determining candidate wool thiopeptide based on a sequence of reference wool thiopeptide; (2) connecting the core peptide coding sequence of the candidate wool thiopeptide with a leader peptide sequence, and connecting the obtained connection product with a vector, so as to obtain a recombinant expression vector; (3) carrying out contact reaction on the recombinantexpression vector, a cell extract and an amino acid premix solution to obtain the candidate wool thiopeptide, and (4) performing screening to obtain the target wool thiopeptide based on different properties of the candidate wool thiopeptide. According to the method, the target wool thiopeptide can be conveniently and quickly obtained by utilizing the cell-free protein synthesis system.
Owner:WUHAN HESHENG TECH CO LTD
A kind of novel lysyl endopeptidase and preparation method thereof
ActiveCN109486800BHas whole enzyme activityReserved functionVector-based foreign material introductionPeptidasesArginineIon exchange
The invention discloses novel lysyl endopeptidase. A suitable flexible linker peptide is connected to an enzyme core peptide, a histidine label and an arginine tail end, while the original enzymatic activity is maintained, nanogram-level residual enzyme in a sample is detected by an ELISA method, and furthermore, novel lysyl endopeptidase sterling is obtained by purification of an ion exchange layer. The invention further provides a preparation method of the lysyl endopeptidase, after 1L of fermentation broth is purified, the recycling amount of 107 mg of the novel lysyl endopeptidase sterlingcan be achieved, and the novel lysyl endopeptidase has industrial potential.
Owner:ZHUHAI JINBAIKANG BIOLOGICAL TECH CO LTD
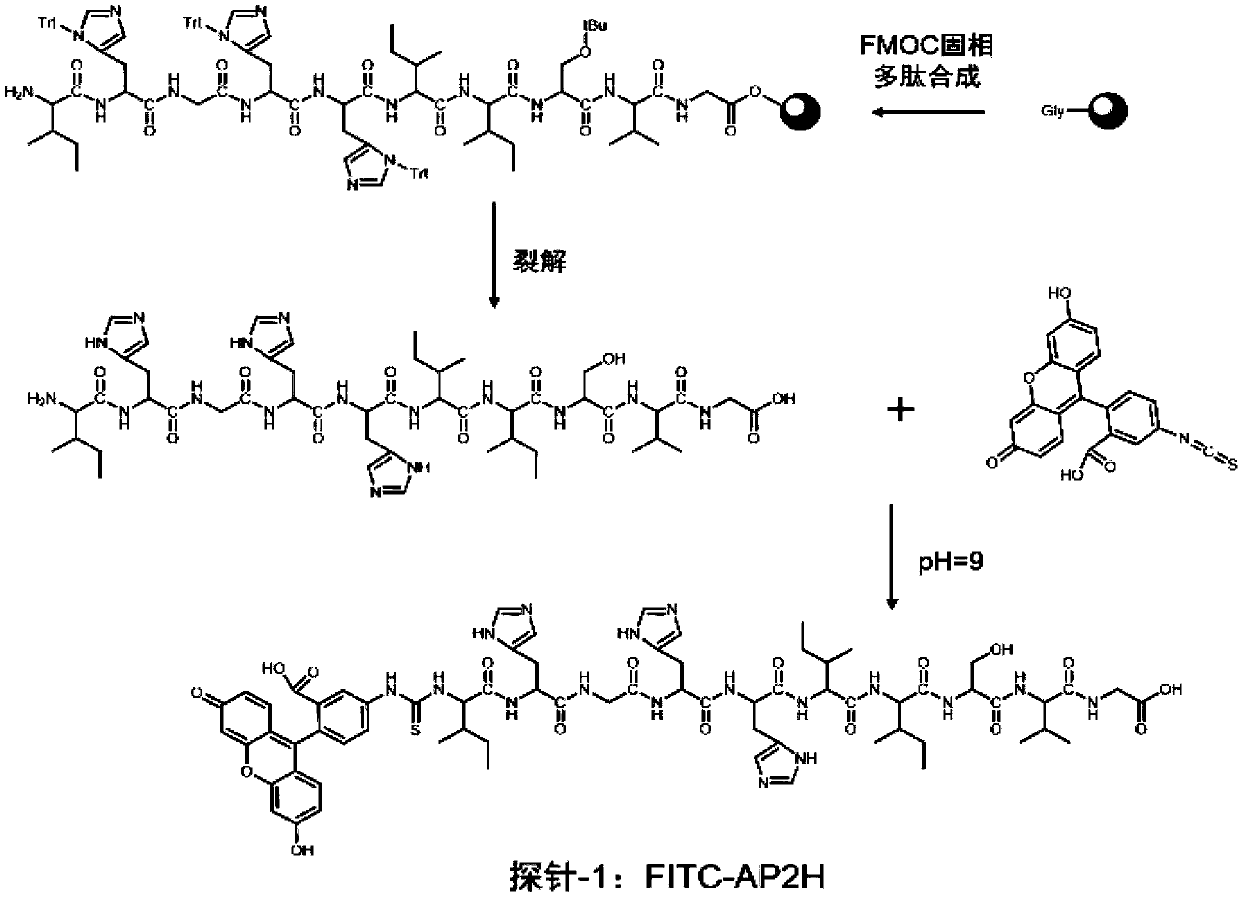
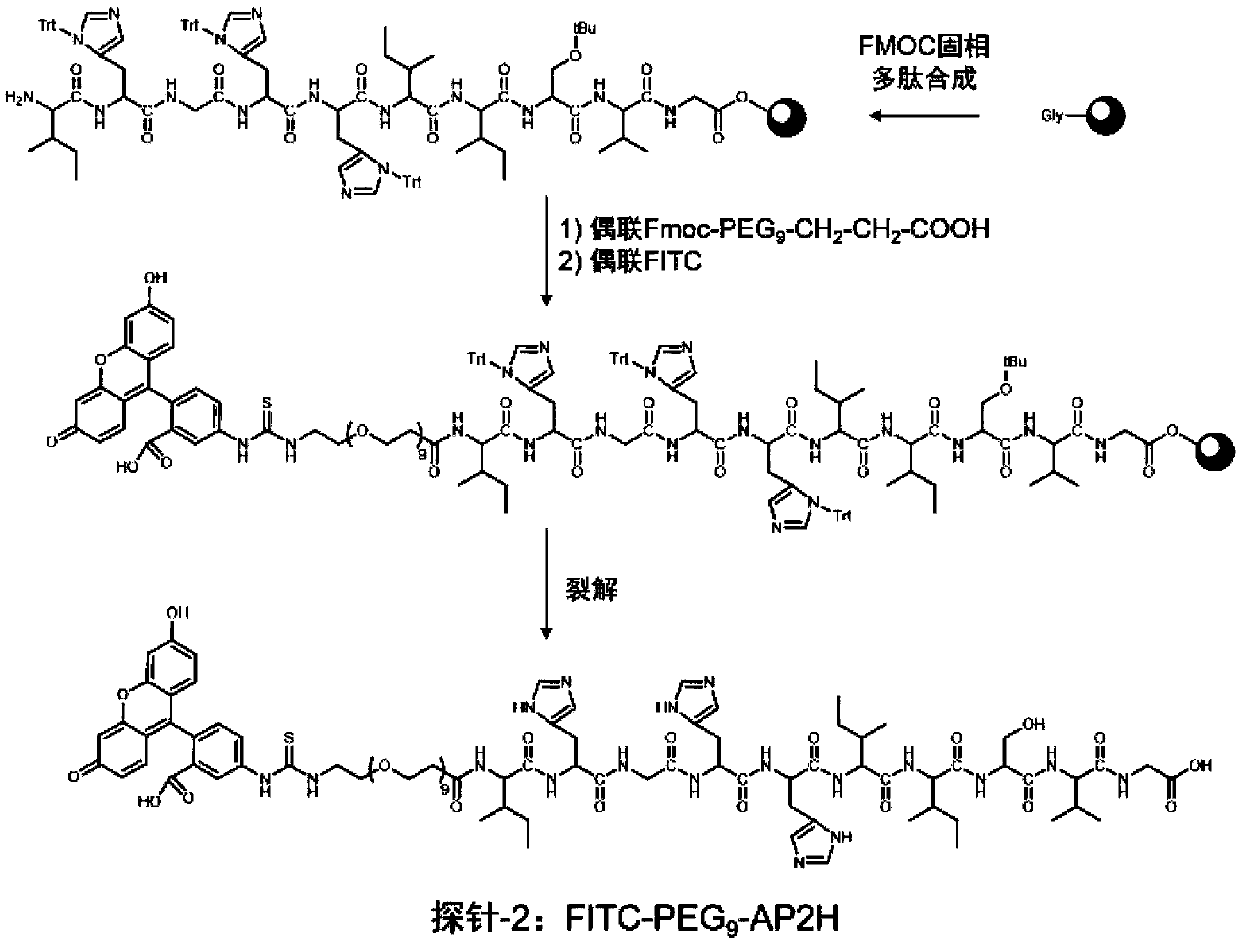
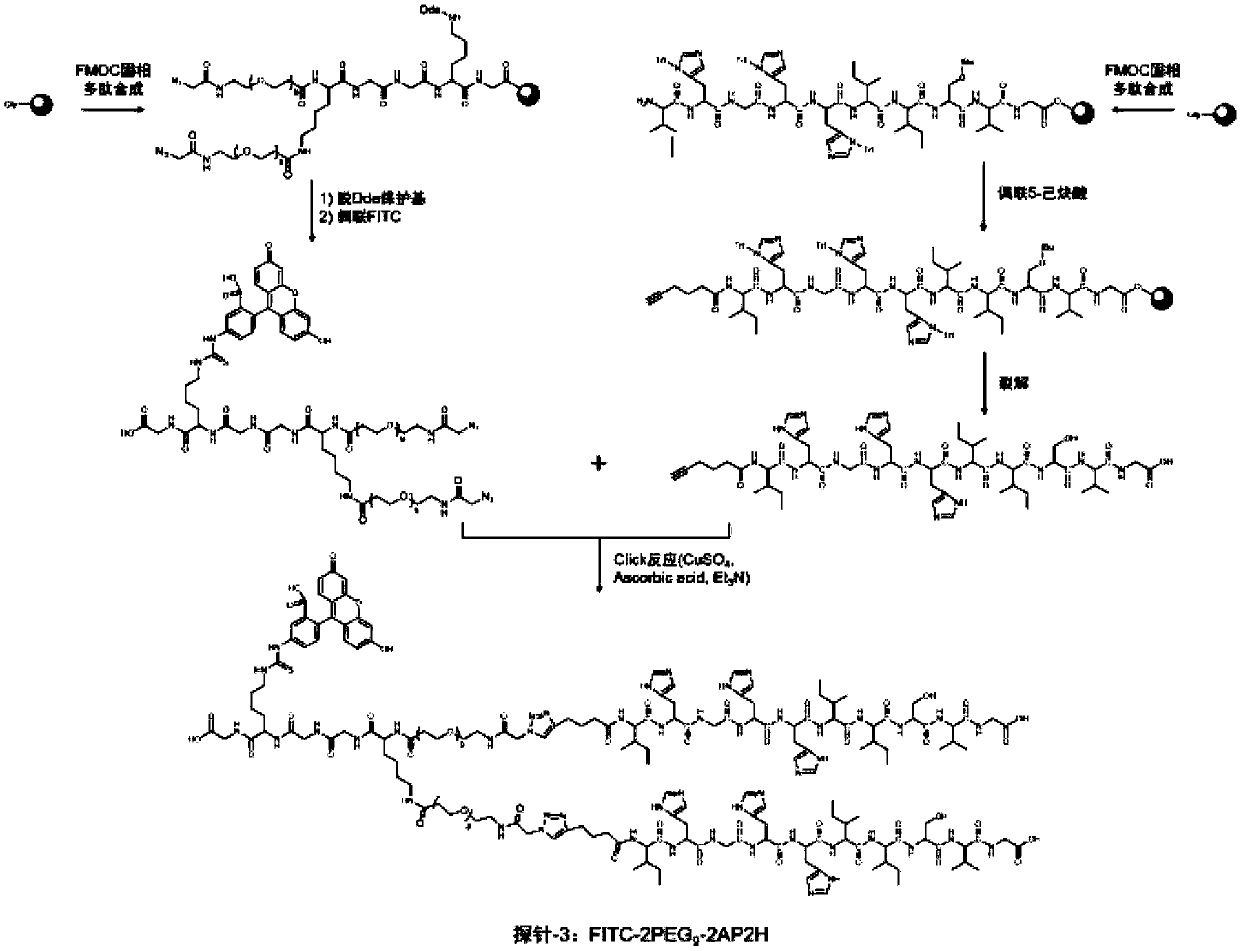
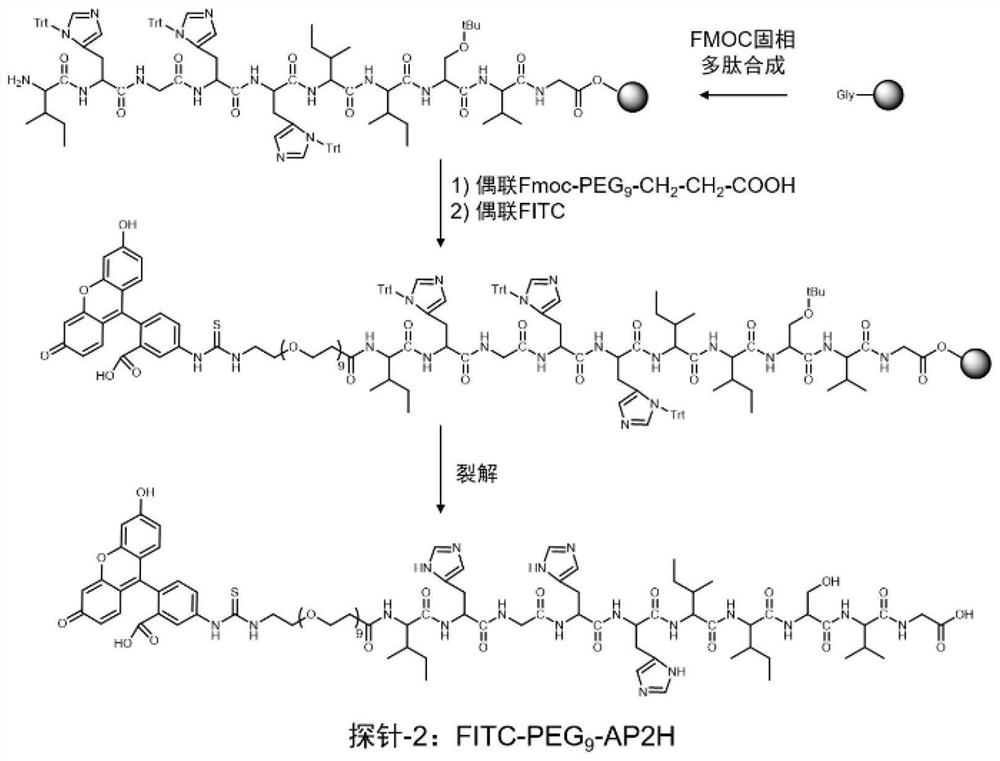
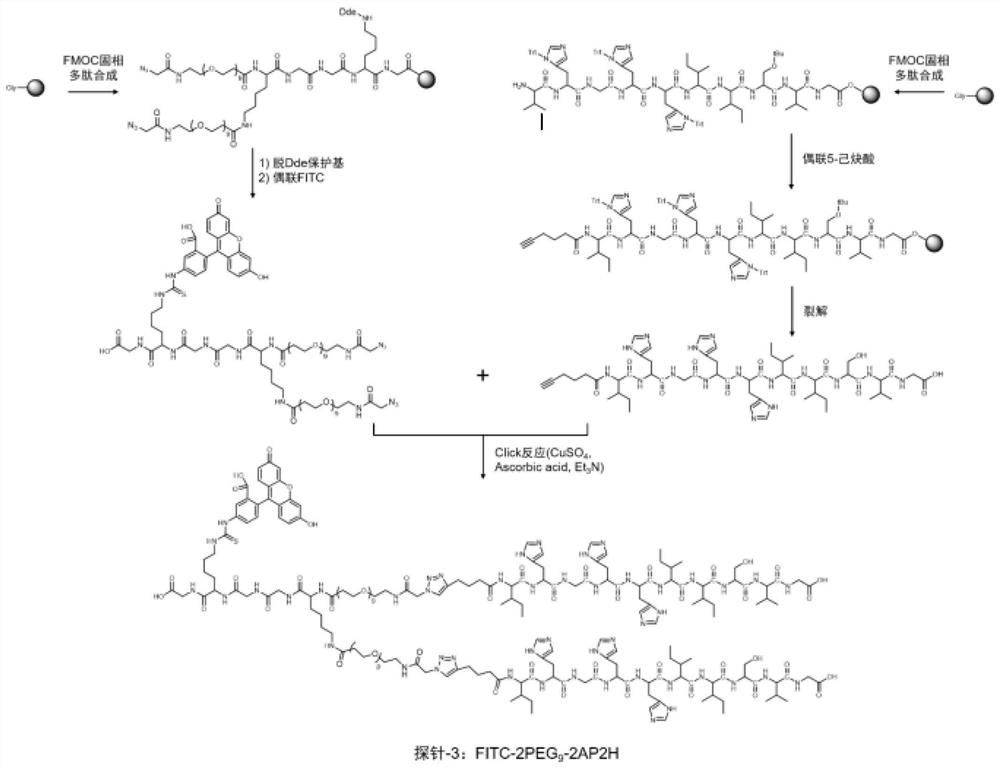
Divalent targeted polypeptide probe and preparation method thereof
ActiveCN110456053AHigh Sensitivity Fluorescence QuantitationImprove hydrophilicityBiological testingBulk chemical productionSolubilityFluorescence
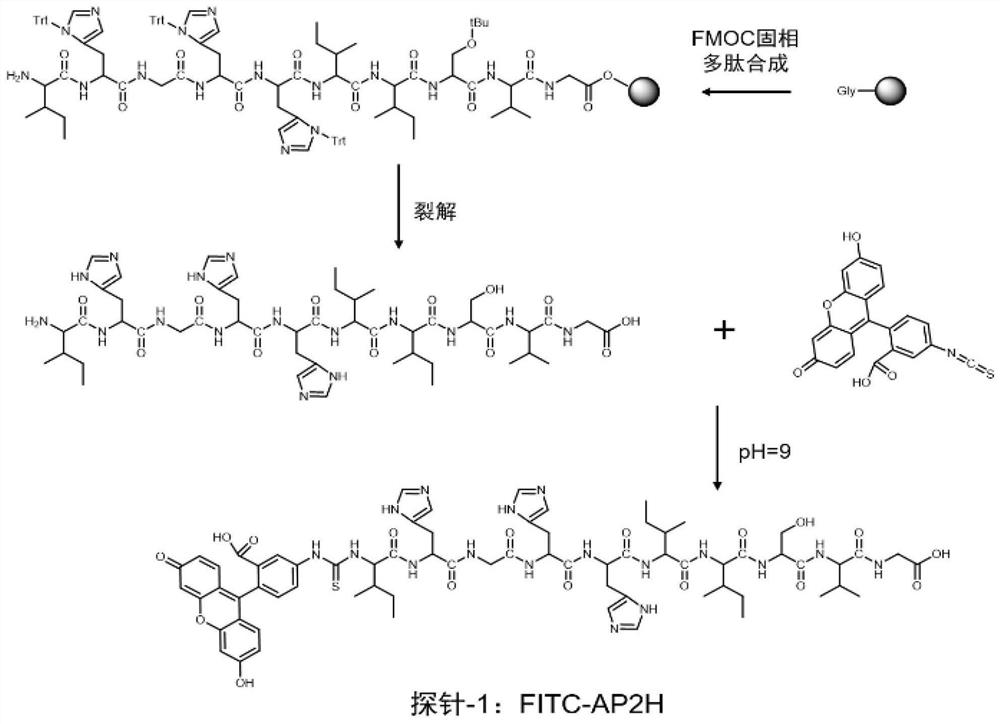
The invention discloses a divalent targeted polypeptide probe and a preparation method and application thereof. The probe comprises A) two tumor specific targeting polypeptide; B) two PEG linking arms; C) skeleton core peptide and D) fluorescence molecules. Each tumor specific targeting polypeptide is connected with the PEG linking arm via a group as shown in the formula I; the amino acid sequenceof the skeleton core peptide is KGGKG; the skeleton core peptide is coupled to the two tumor specific targeting polypeptide via the PEG linking arms; and the fluorescence molecules are connected to the skeleton core peptide via chemical bonds. According to the multivalent targeted probe FITC-2PEG9-2AP2H, two targeted ligands AP2H are coupled to the skeleton core peptide via PEG chains, the PEG chains can improve the dissolvability of the targeted polypeptide probe in an aqueous solution as well as the flexibility of the probe, and thus, the probe can identify and be combined with a target object.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
Derivatives of laspartomycin and preparation and use thereof
The present invention provides laspartomycin core peptides, laspartomycin core peptide derivatives, antimicrobial laspartomycin derivatives, methods for making laspartomycin core peptides, methods for making laspartomycin core peptide derivatives, methods for making antimicrobial laspartomycin derivatives, pharmaceutical compositions of antimicrobial laspartomycin derivatives, methods of inhibiting microbial growth and methods for treating and / or preventing microbial infections in a subject.
Owner:BIOSOURCE PHARM INC
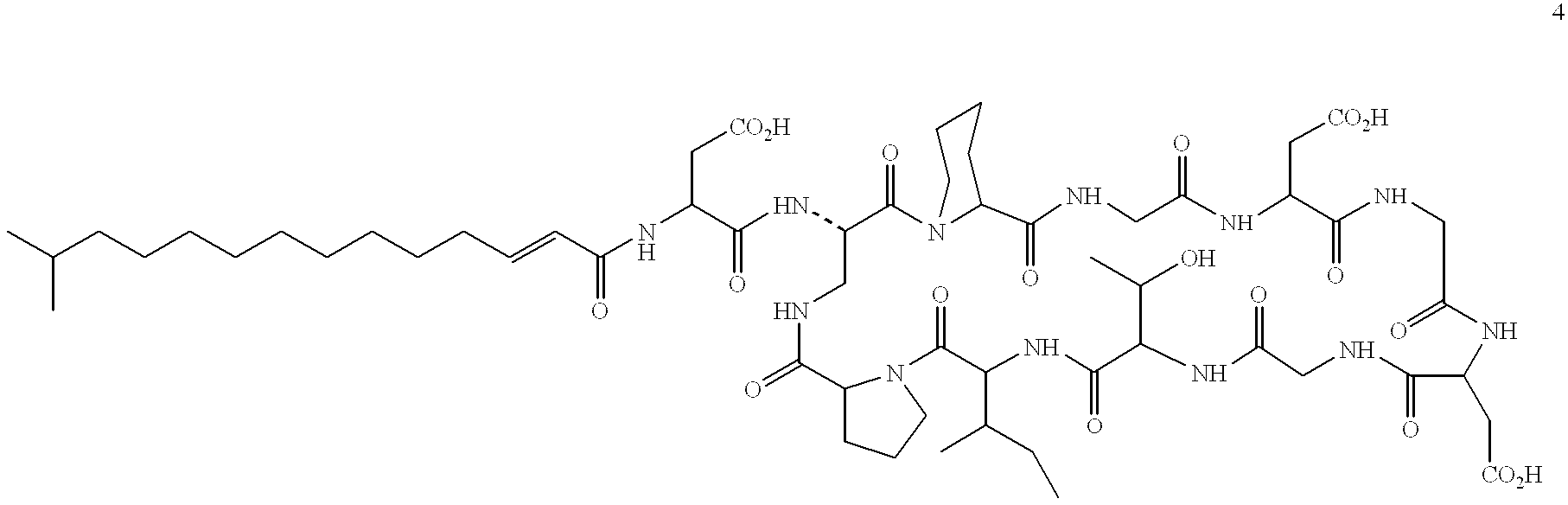
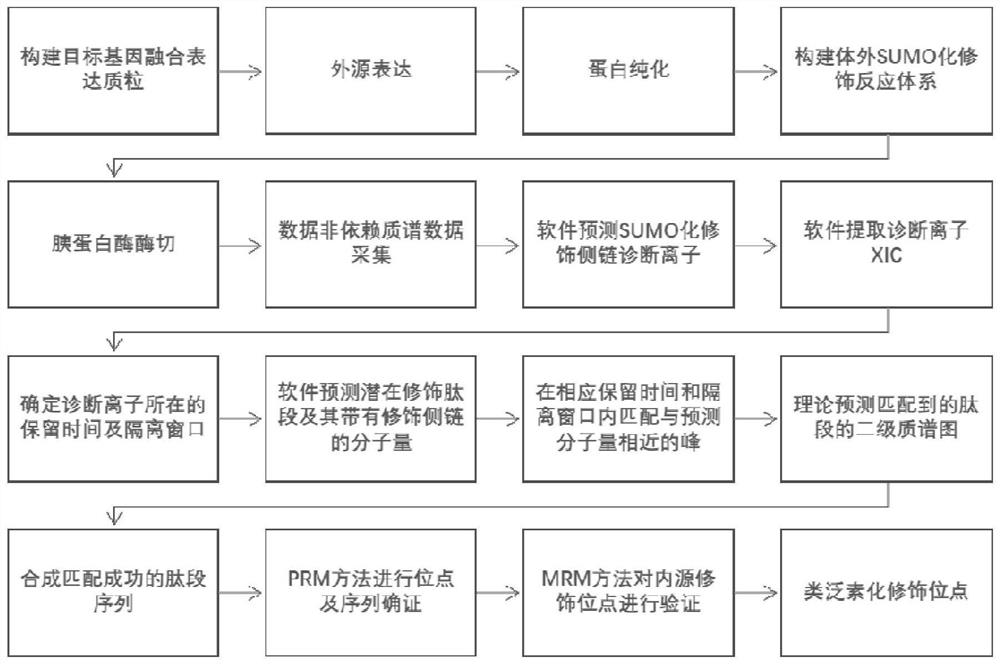
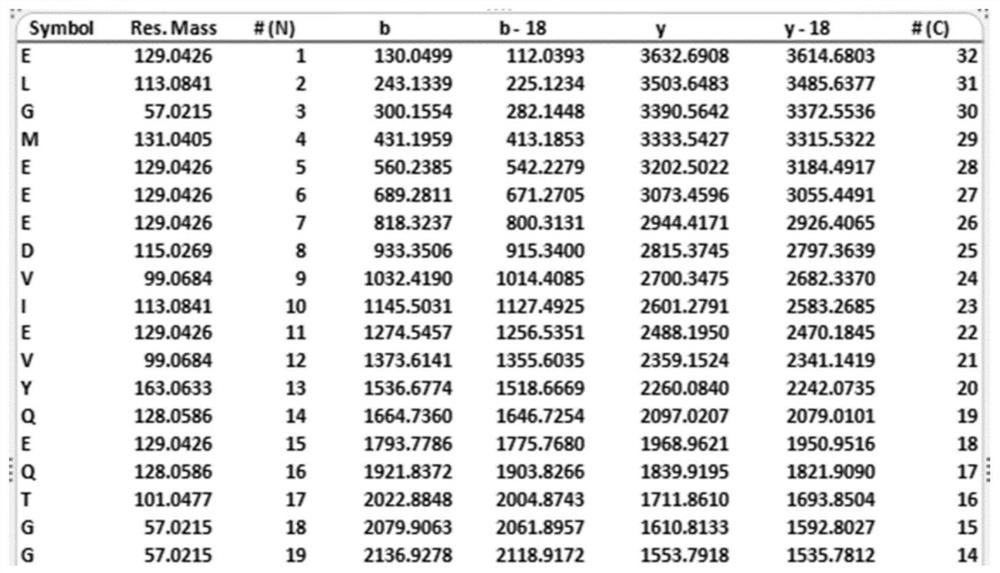
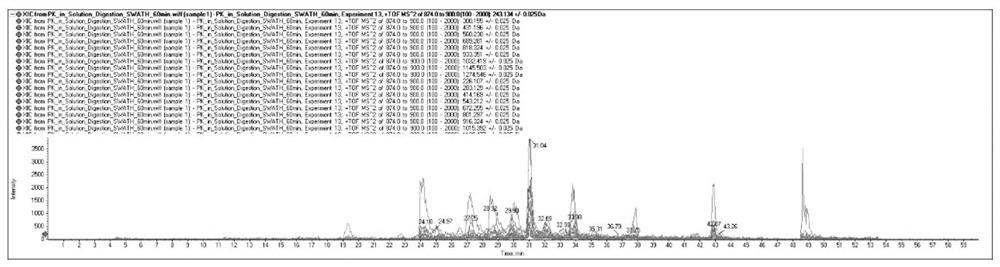
Protein ubiquitination modification site detection method based on high-precision mass spectrum and application
PendingCN113848259AAvoid missingLow costComponent separationBiological testingChemical synthesisEnzyme digestion
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Systematic discovery, maturation and extension of peptide binders to proteins
Owner:ROCHE SEQUENCING SOLUTIONS INC
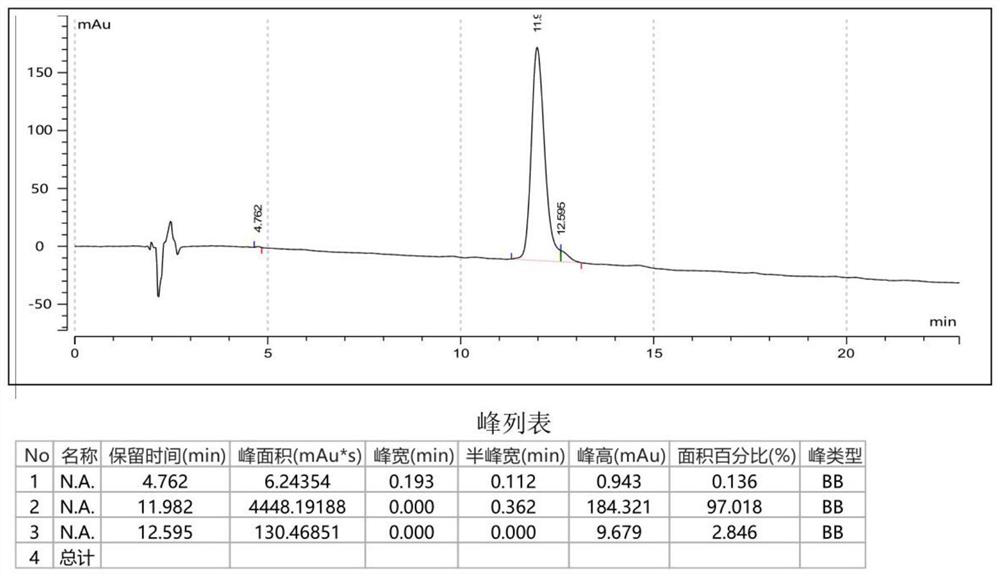
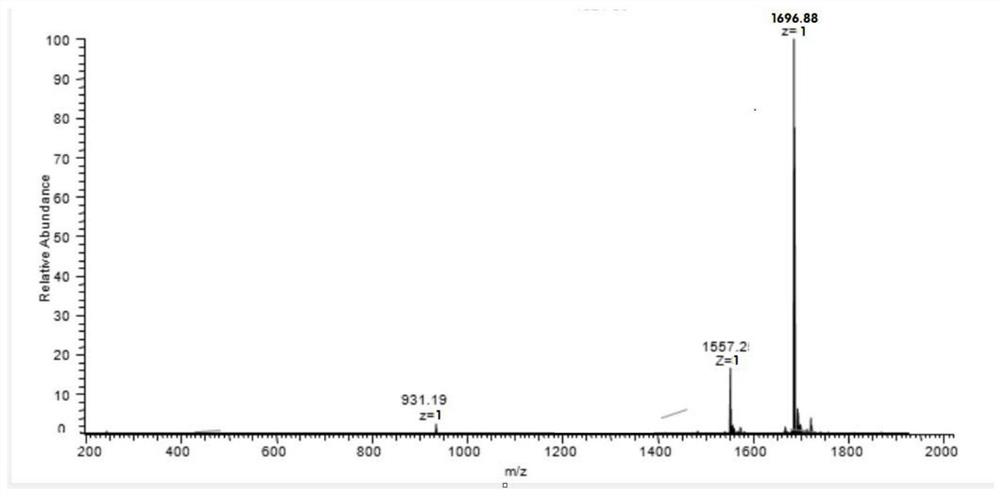
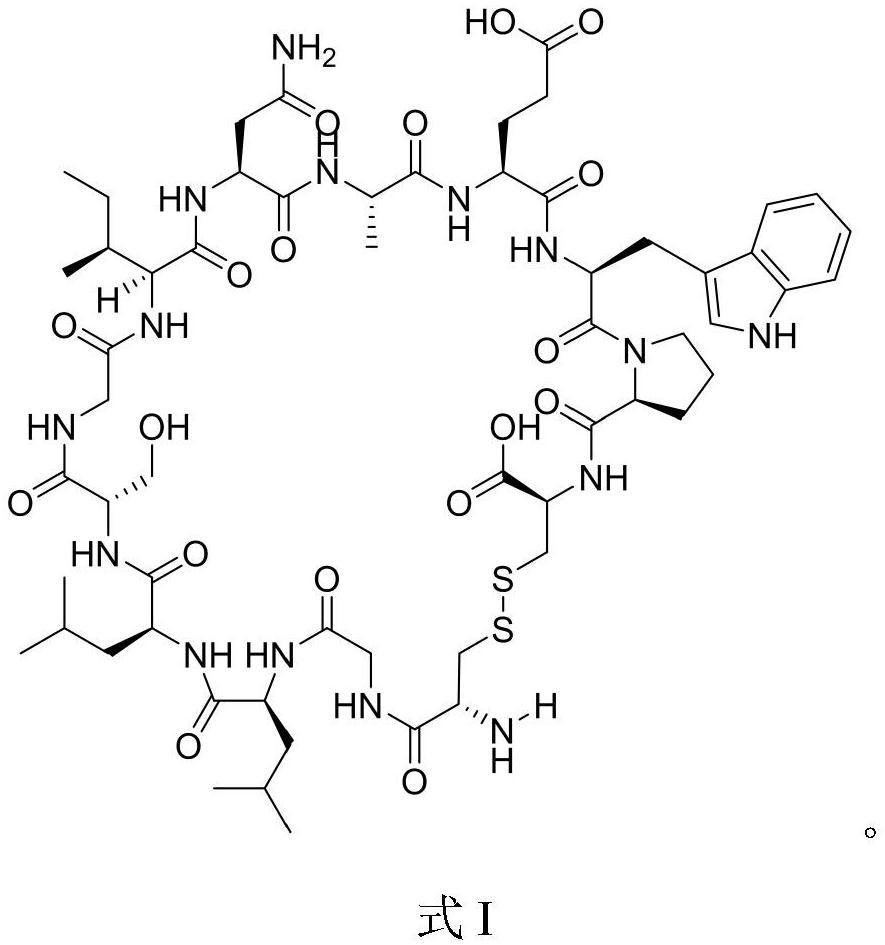
Core-peptide composition with physiological activity and its preparation method and application
ActiveCN101240015AKeep aliveTo achieve the purpose of removing impuritiesPeptide/protein ingredientsPeptide preparation methodsConchBiotechnology
The invention relates to a natural composite with biological activity extracted from conch, and application in the pharmaceutical field of the same, pertains to biological and pharmaceutical technical fields. The invention protects a natural composite with biological activity, preparation and application thereof. The invention is extracted from marine organism of conch, has a unique biological activity, can notably content of primotest in PADAM patient's blood.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
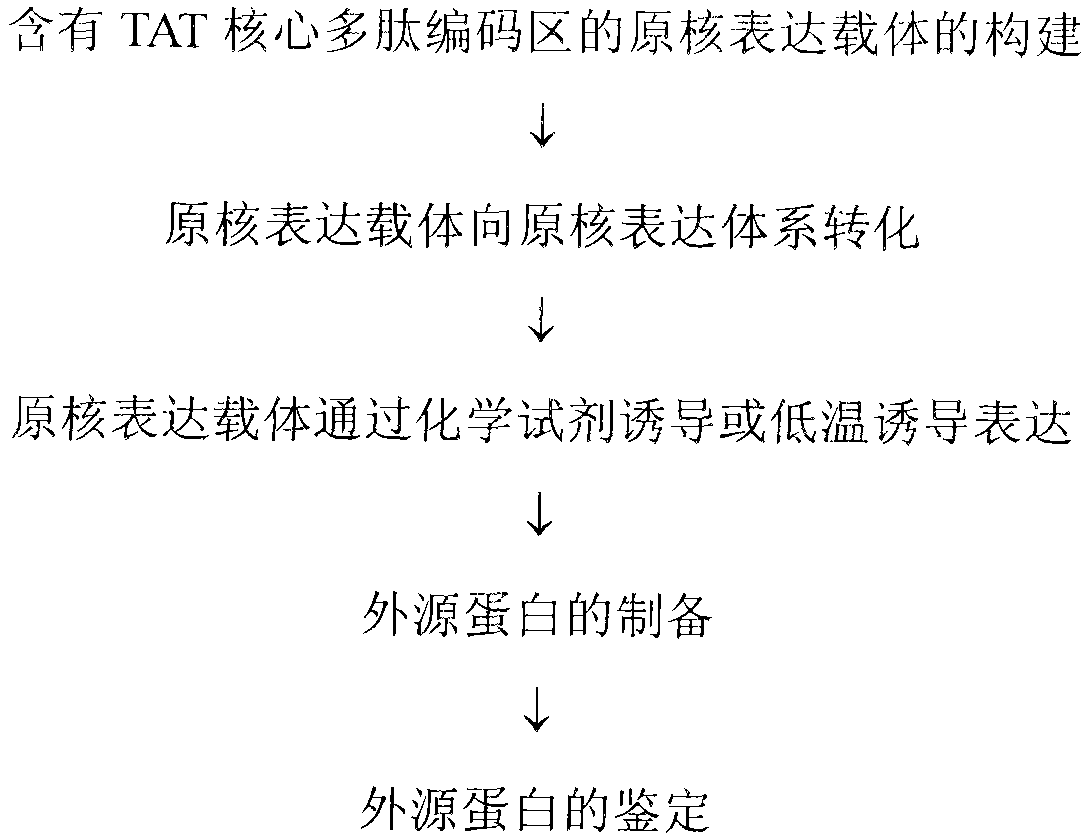
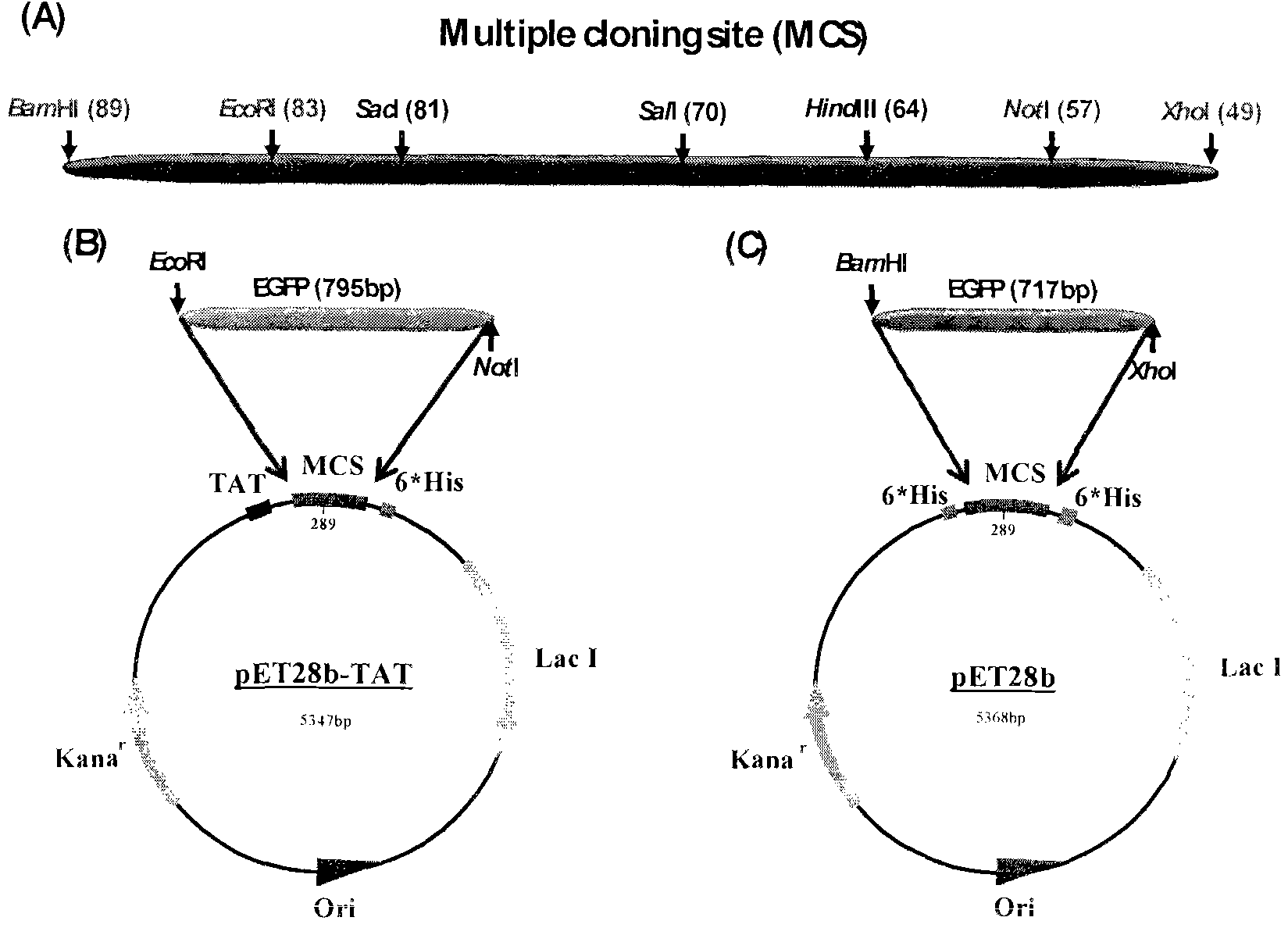
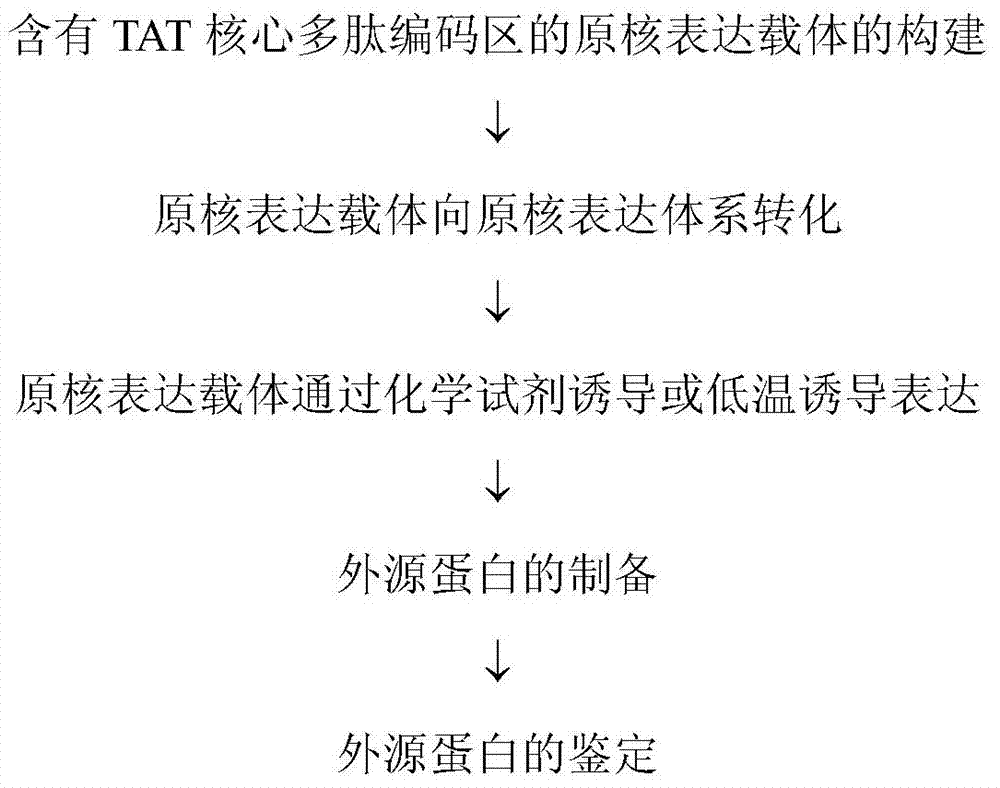
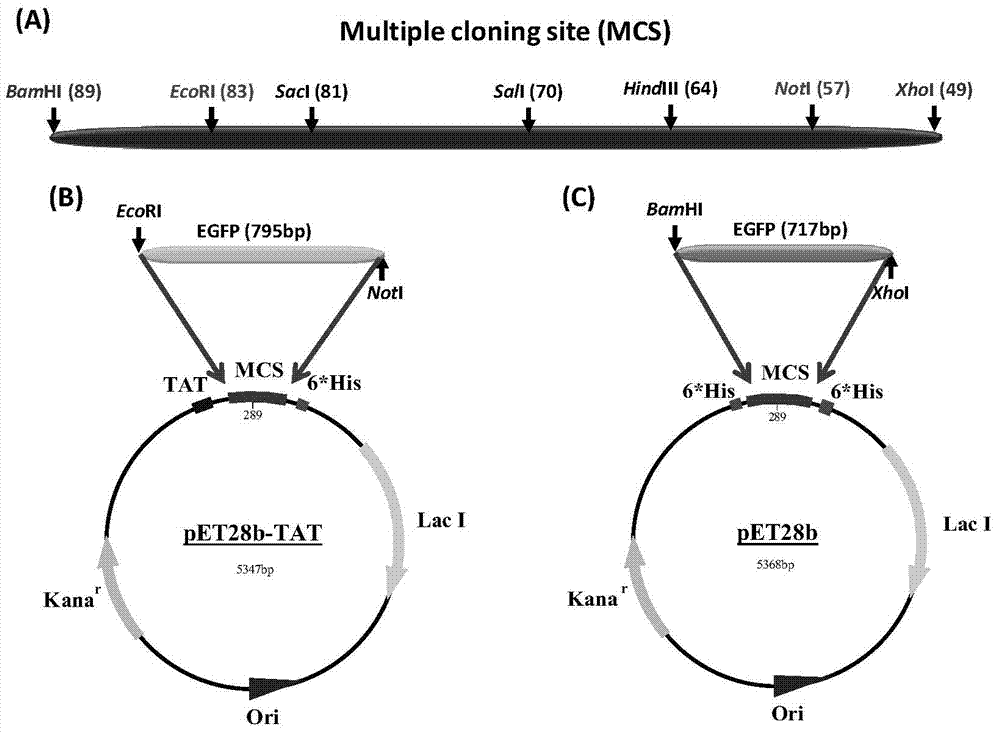
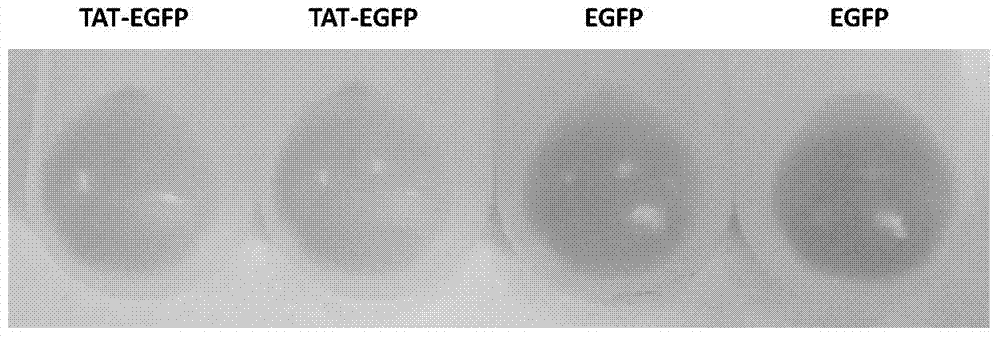
Method and special expression vector thereof for promoting solubility expression of foreign protein by TAT (Trans-activator transduction) core peptide
InactiveCN102146417BSolve some difficult problems that are difficult to express efficientlyLow costFermentationVector-based foreign material introductionSolubilityTotal protein
The invention discloses a method and a special expression vector thereof for promoting a prokaryotic expression system to efficiently perform solubility expression of foreign protein by TAT (Trans-activator transduction) core peptide. The special expression vector is a prokaryotic expression vector containing one or more TAT core polypeptide coding regions. The method comprises the following steps of: (1) inserting foreign genes into the special expression vector to obtain the prokaryotic expression vector containing TAT core polypeptide coding regions and foreign genes; (2) converting the prokaryotic expression vector into the prokaryotic expression system; (3) performing inducible expression of the prokaryotic expression vector; and (4) preparing foreign protein. Proven by detection, the expression product mainly exists in a soluble form in host bacteria lysis supernatant, and accounts for 25-30% of the total protein in the lysis supernatant, so that high yield foreign protein can be obtained and a big amount of foreign protein with biological activity can be prepared.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
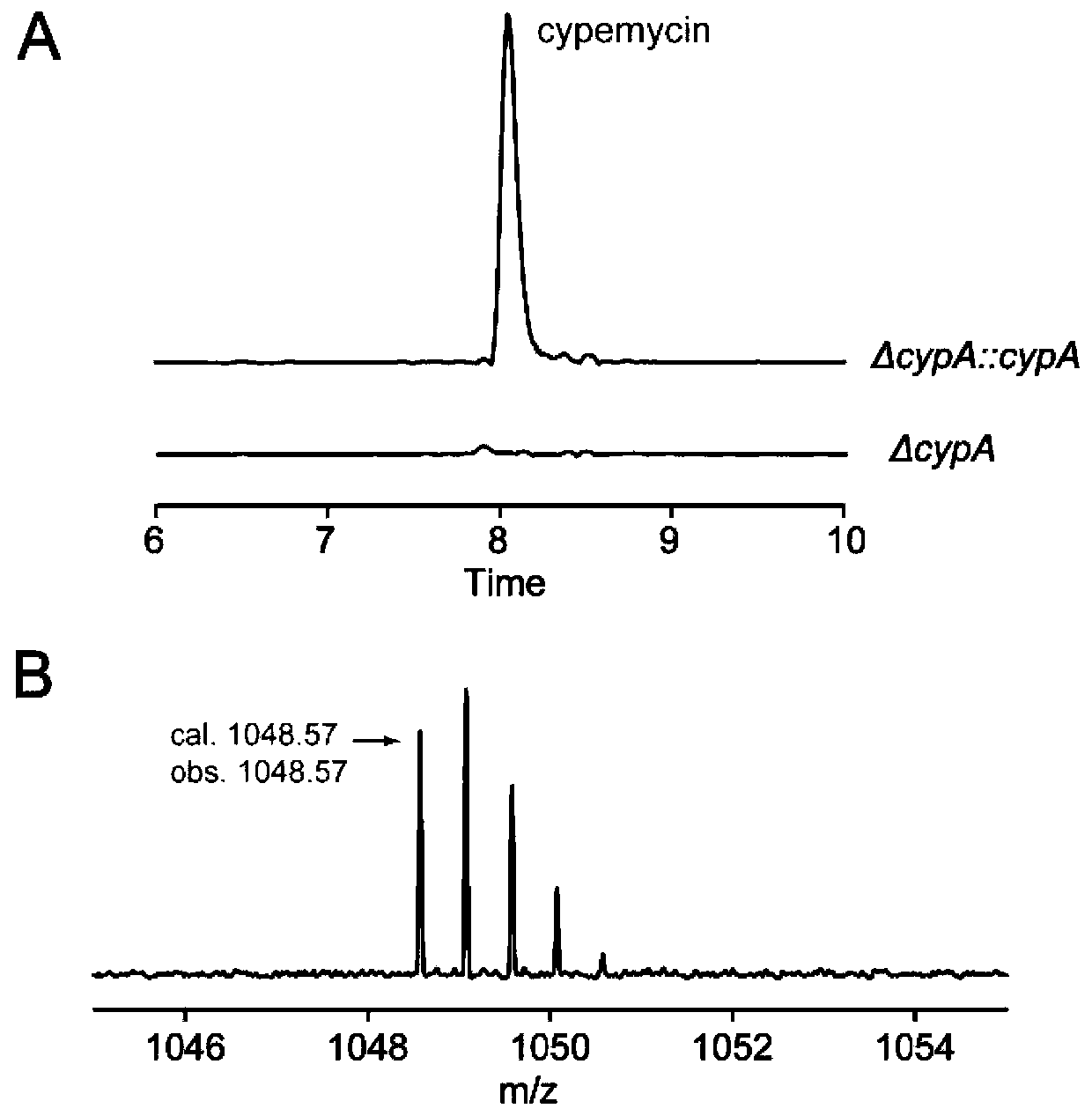
Cypemycin precursor peptide mutant and application thereof and prepared cypemycin analogues
PendingCN111454338AOvercome very few typesOvercome the shortcomings of the existing septomycin analogues with few typesAntibacterial agentsBacteriaThreonineTyrosine
The present invention discloses a cypemycin precursor peptide mutant and an application thereof and prepared cypemycin analogues. The mutant is prepared by mutating one or more threonines in a core peptide to serine, lysine, alanine or leucine; and / or, mutating one or two glutamines in the core peptide to glutamic acid; and / or, mutating serine in the core peptide to alanine or tyrosine; and / or, mutating valine at a 12nd and / or 21st position of a sequence shown in SEQ ID NO.1 to lysine, alanine, isoleucine, or cysteine; and / or, mutating cysteine at a 19th position of the sequence shown in the SEQ ID NO.1 to serine or tyrosine; and / or, mutating cysteine at a 33rd position and / or proline at a 36th position of a sequence shown in SEQ ID NO.2 to alanine. A series of cypemycin analogues can be prepared by using the mutant and provide a basis for screening compounds with higher activity.
Owner:南京凯沫比尔生物科技有限公司
Multifunctional anti-cancer nano material based on polypeptide-rare earth nanocrystals and preparation method of multifunctional anti-cancer nano material
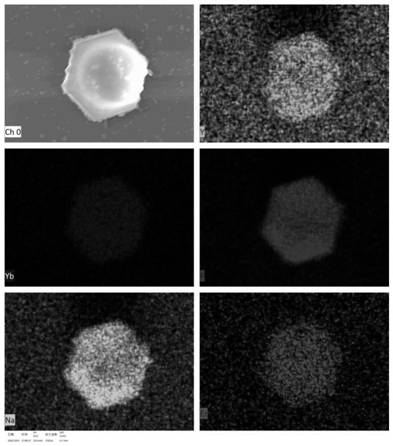
PendingCN114177310AOvercoming multidrug resistanceSolve the disadvantage of not being able to track fluorescenceOrganic active ingredientsEnergy modified materialsMicrosphereCombined treatment
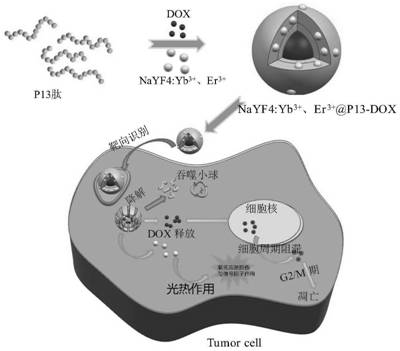
The invention discloses a multifunctional anticancer nano material based on polypeptide-rare earth nanocrystals, which is characterized in that the nano material is a hydrophilic nano microsphere of polypeptide entrapped drug adriamycin and NaYF4: Yb < 3 + >, Er < 3 + > nanocrystals; wherein the hydrophobic end of the P13 peptide is combined with the medicine doxorubicin and the NaYF4: Yb < 3 + > and Er < 3 + > nanocrystalline in a non-covalent bond form to form a hydrophobic core; and the hydrophilic end of the P13 peptide is used as a targeting end to form a hydrophilic shell. According to the invention, polypeptide is used as a carrier for entrapment of drug adriamycin, and drug inhibition and a PDT method are combined to kill tumor cells in a manner of entrapment of NaYF4: Yb < 3 + >, Er < 3 + > nanocrystals. According to the invention, a remarkable super-addition (1 + 1 > 2) effect is generated in a synergistic treatment mode, and the effect is remarkably enhanced compared with that of any single therapy; meanwhile, the multi-mode combined treatment mode can effectively overcome the multi-drug resistance of the tumor; in addition, the defect that the drug loading system cannot be subjected to fluorescence tracking due to the fact that the polypeptide has no fluorescence characteristic is overcome.
Owner:HUBEI UNIV
Polypeptide analogue as well as preparation method and application thereof
PendingCN114716515ANot easily oxidizedImprove stabilityCosmetic preparationsPeptide/protein ingredientsDisulfide bondingLong chain fatty acid
The invention discloses a polypeptide analogue and a preparation method and application thereof, a core peptide sequence of the polypeptide analogue is OA-GL12, and the core peptide is modified by one or more of the following steps: 1) extending at the N terminal of the core peptide, extending peptide is Cys, and the extending peptide Cys and the core peptide Cys form a disulfide bond; (2) carrying out O-glycosylation modification on the core peptide; 3) carrying out fatty acid modification on the N terminal of the core peptide; and 4) fusing SYN-AKE at the tail end of the core peptide C. The Cyclic-GC12 subjected to cyclization modification is not easy to oxidize and is good in stability; glyco-GC12 subjected to glycosylation modification has better skin absorptivity, so that the Glyco-GC12 can be used for preparing the skin care product; the transdermal effect of the long-chain fatty acid modified Pal-GC12 is enhanced, and the stability is better; the SYN-AKE-GC12 fused by the SYN-AKE-GC12, the SYN-AKE-GC12, the SYN-AKE-GC12 and the
Owner:深圳深创生物药业有限公司
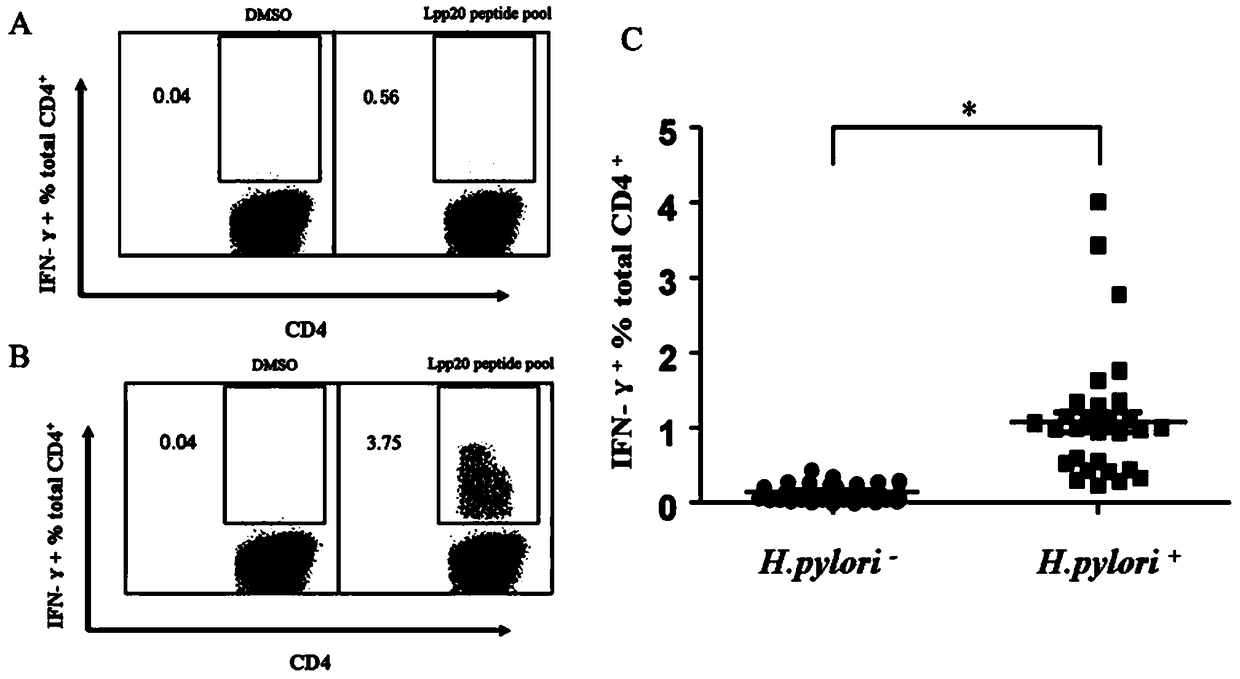
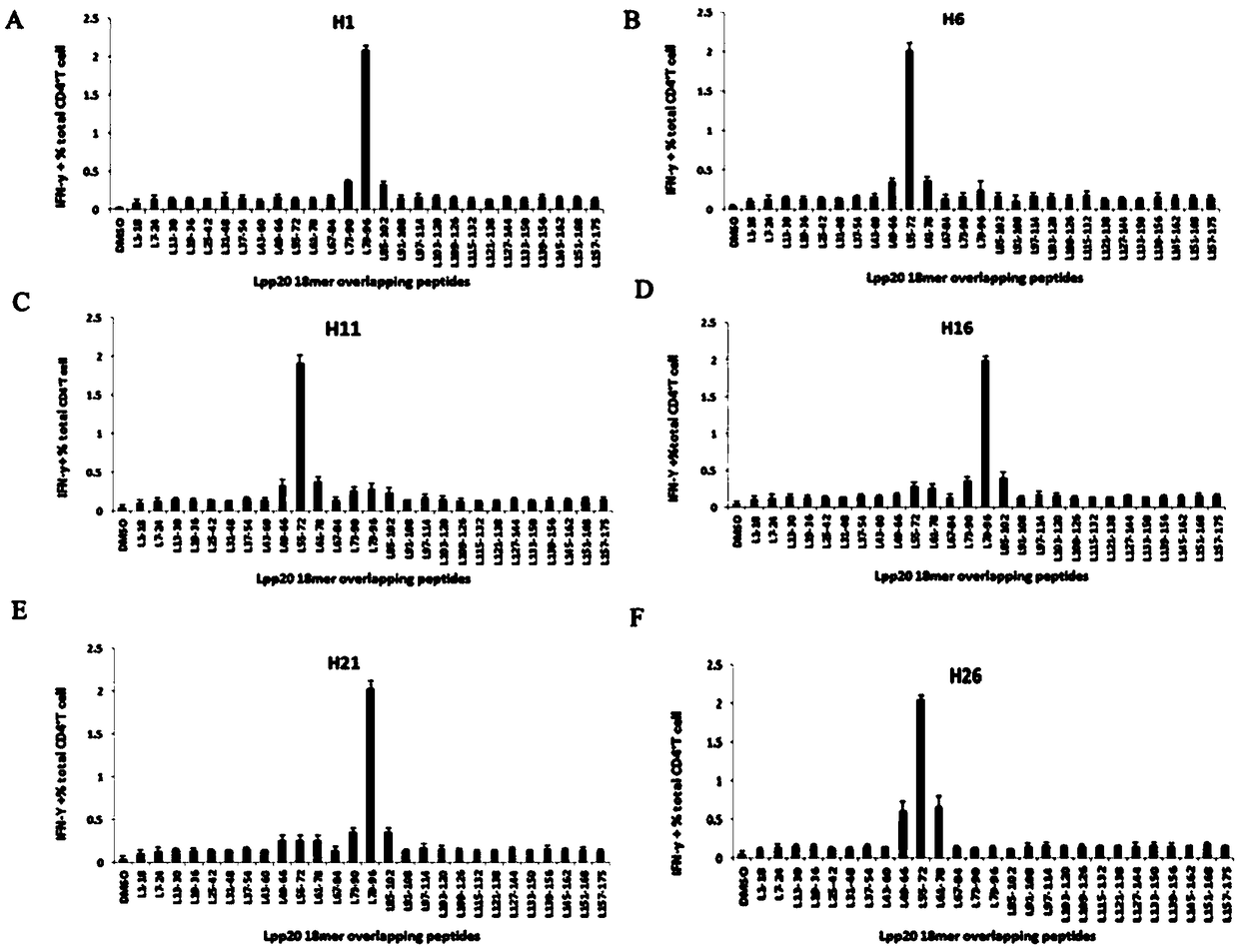
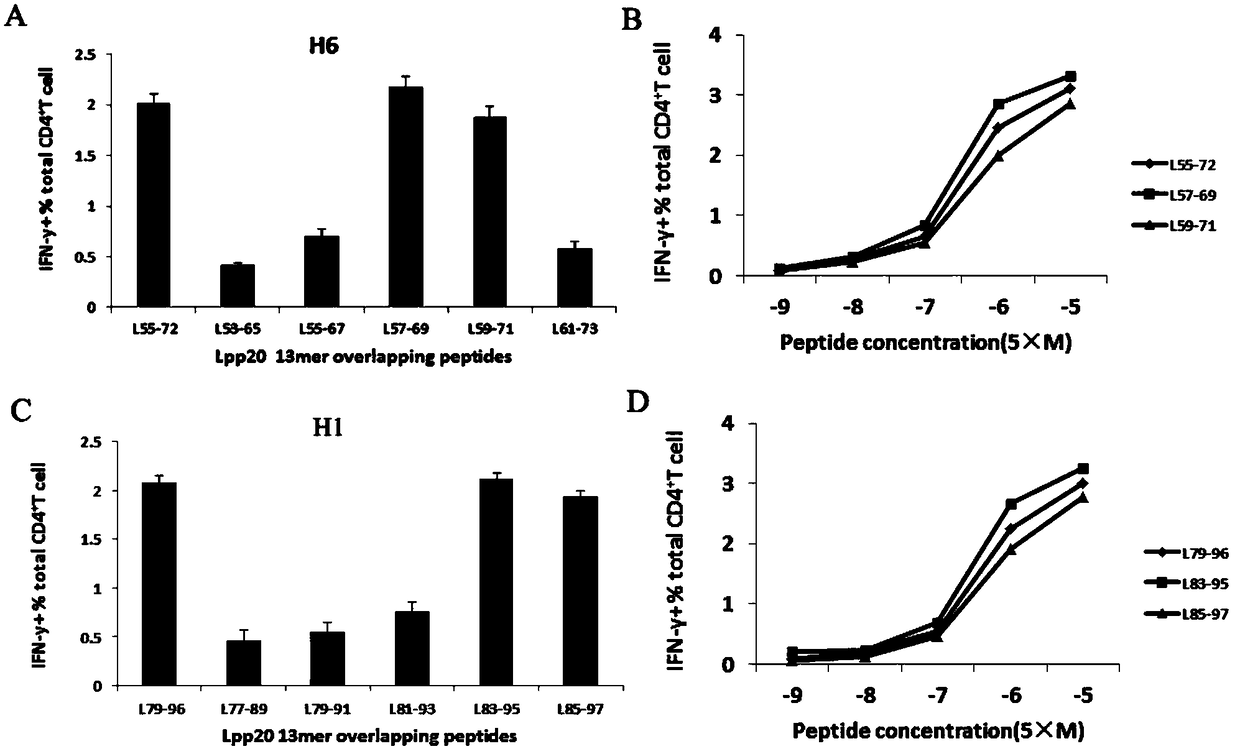
Helicobacter pylori immunodominant epitope peptide L79-96 and application of same
The invention relates to HLA-restricted CD4<+> T cell epitope immunodominant peptide and core peptide of Helicobacter pylori Lpp20, which have structures represented as the SEQ ID No.10 and SEQ ID No.30; the proper CD4<+> T cell epitopes (SEQ ID No.10 and SEQ ID No.30) in the invention are reliable and accurate; what is more, it can be evaluated that the identified epitope belongs to whether immunodominance or subdominance, which has advantages on design of epitope vaccines.
Owner:SOUTHERN MEDICAL UNIVERSITY
A novel lysine-specific endonuclease and its preparation method
ActiveCN109439643BReserved functionIncrease productionFermentationVector-based foreign material introductionAssayPolyhistidine-tag
The invention discloses novel lysyl endopeptidase. An enzyme core peptide is connected with histidine tags through an appropriate flexible linker peptide, so that nanogram residual enzyme in a samplecan be detected by an ELISA (enzyme-linked immunosorbent assay) method; and total activity of wild lysyl endopeptidase is ensured. At the same time, the invention further provides a preparation methodof the lysyl endopeptidase. Compared with a gene engineering modification and fermentation technology, the preparation method has the benefit of high yield; a recovery amount of 857mg novel lysyl endopeptidase can be obtained after purification of 1L fermentation liquid; and the method has an industrial potential.
Owner:ZHUHAI JINBAIKANG BIOLOGICAL TECH CO LTD
Application of TAT core peptide fragment in preparing efficiently and solubly expressed exogenous protein
InactiveCN103865945ASolve some difficult problems that are difficult to express efficientlyLow costDepsipeptidesVector-based foreign material introductionProkaryotic expressionExogenous protein
The invention relates to an application of a TAT core peptide fragment in preparing an efficiently and solubly expressed exogenous protein and discloses a method for efficiently and solubly expressing the exogenous protein by using a prokaryotic expression system under promotion of the TAT core peptide fragment, and a special expression vector. By implementation of the method and the special expression vector, not only can the soluble exogenous protein be obtained, but also the exogenous protein with high yield can be obtained, so that a foundation is laid and a key technology is provided for preparing the exogenous protein with bioactivity on large scale, and the problem that the exogenous protein is difficult to efficiently express in the fields of bioengineering, research and development of drugs and the like is solved, and thus the cost of separating and purifying the exogenous protein in the following steps can be remarkably saved.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Multiple antigenic agents and methods for using the same
The present invention provides multiple antigenic agents compositions and the use thereof to prevent or treat viral infections. The multiple antigenic agents of the invention contain at least one of a B cell determinant, a T cell determinant, or a targeting molecule attached to a core peptide composed of Lys-Gly repeats.
Owner:WISTAR INST THE A CORP OF PA
Methods and compositions for derepression of IAP-inhibited caspase
InactiveUS20050159359A1Promote apoptosisReduce severityNervous disorderPeptide/protein ingredientsDerepressionApoptosis
Owner:THE BURNHAM INST
A kind of bivalent targeting polypeptide probe and preparation method thereof
ActiveCN110456053BHigh Sensitivity Fluorescence QuantitationImprove hydrophilicityPeptidesBiological testingTumor specificTargeting ligands
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
Biological fermentation preparation method of semaglutide core peptide chain
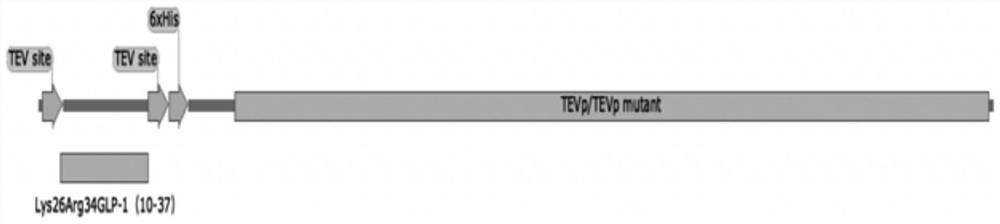
PendingCN114457099AReduce enzyme digestion stepsResidue reductionBacteriaMicroorganism based processesFusion Protein ExpressionEnzyme digestion
The invention relates to the technical field of biological engineering, in particular to a biological fermentation preparation method of a semaglutide core peptide chain, and the core peptide chain is Lys26Arg34GLP-1 (9-37), Lys26Arg34GLP-1 (10-37) or Lys26Arg34GLP-1 (11-37). The specific method comprises the following steps: coupling a core peptide chain with an incision enzyme or a mutant thereof required by the fusion protein to obtain a fusion protein DNA sequence, then constructing an expression vector of the fusion protein, loading an expression cell, expressing the fusion protein to obtain an inclusion body, and carrying out renaturation purification and enzyme digestion to obtain a product. Compared with simultaneous enzyme digestion and renaturation, the method has the advantages of no need of additional addition of incision enzyme, low cost, economy, high efficiency and environmental friendliness.
Owner:JIANGSU ALPHA PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com