Polypeptide analogue as well as preparation method and application thereof
A technology for analogs and polypeptides, applied in the field of polypeptide analogs and their preparation, to achieve the effects of reducing muscle contraction, not easy to be oxidized, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
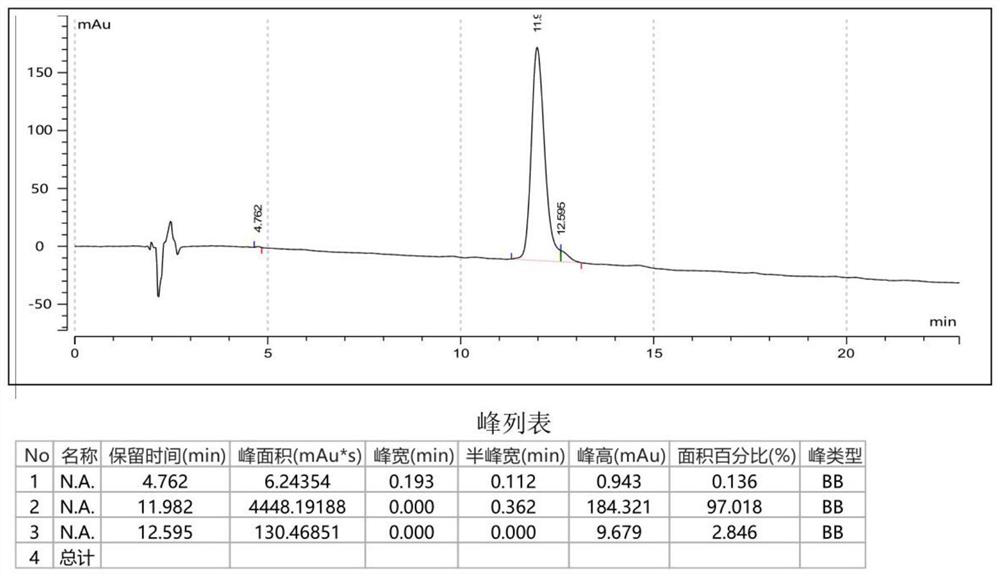
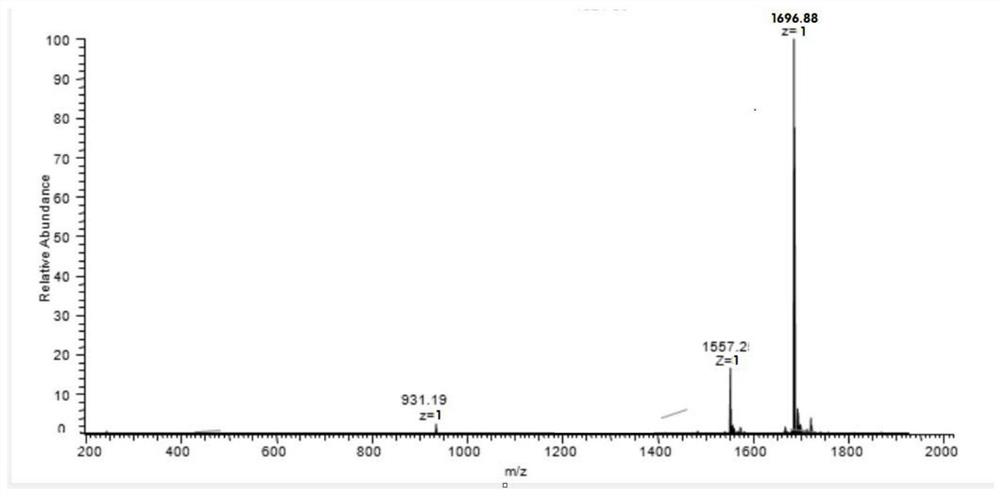
[0048] Example 1: Preparation of Cyclic-GC12
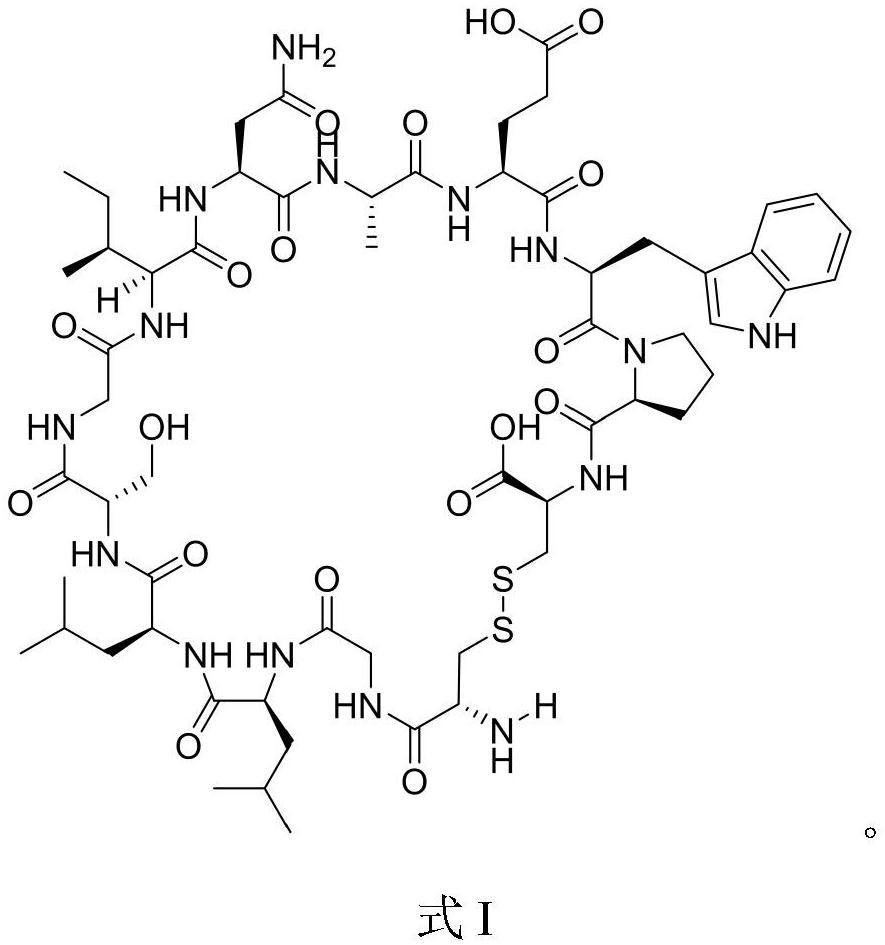
[0049] Peptide sequence of Cyclic-GC12: CGLLSGINAEWPC (Disulfide cyclic peptide), molecular weight: 1360.57, and its structural formula is as follows:
[0050]
[0051] The preparation of Cyclic-GC12, the specific steps are as follows:
[0052] 1.1. Weigh 2.5 g (1 mmol) of H-Cys(Trt)-2-Chlorotryl Resin resin (Sub=0.40 mmol / g) into the reaction column, wash with DMF 3 times, and swell with DMF for 30 minutes. Weigh 2.6 g (6 mmol) of Fmoc-Pro-OH and 0.9 g (6.6 mmol) of HOBt, dissolve in DMF, add 1.2 g of DIC (9 mmol) in an ice-water bath at 0°C, activate for 5 minutes, add to the reaction column, and react for 2 hours. , the ninhydrin test result is negative (resin is colorless and transparent), then use DBLK (20% hexahydropyridine / DMF) to remove the Fmoc protecting group, then repeat the above operation, according to the sequence coupling Fmoc-Trp(Boc)- OH, Fmoc-Glu(OtBu)-OH, Fmoc-Ala-OH, Fmoc-Asn(Trt)-OH, Fmoc-Ile-OH, Fmoc-Gl...
Embodiment 2
[0054] Example 2: Preparation of Glyco-GC12
[0055] The peptide sequence of Glyco-GC12: GLLS(O-β-D-glucose) GINAEWPC, the molecular weight is: 1421.59, and its structural formula is as follows:
[0056]
[0057] The preparation of Glyco-GC12, the specific steps are as follows:
[0058] 2.1. Weigh 2.5 g (1 mmol) of H-Cys(Trt)-2-Chlorotrityl Resin resin (Sub=0.40 mmol / g) into the reaction column, wash with DMF 3 times, and swell with DMF for 30 minutes. Weigh 2.5 g (6 mmol) of Fmoc-Pro-OH and 0.9 g (6.6 mmol) of HOBt, dissolve them in DMF, add 1.2 g of DIC (9 mmol) in an ice-water bath at 0°C, activate for 5 minutes, add to the reaction column, and react for 2 hours. The Fmoc protecting group was then removed with DBLK. Repeat the above operation, according to the sequence coupling Fmoc-Trp(Boc)-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Ala-OH, Fmoc-Asn(Trt)-OH, Fmoc-Ile-OH, Fmoc-Gly -OH, Fmoc-Ser(O-β-D-glucose)-OH, Fmoc-Leu-OH, Fmoc-Leu-OH, Boc-Gly-OH, washed with DMF, DCM, methanol i...
Embodiment 3
[0060] Example 3: Preparation of Pal-GC12
[0061] Peptide sequence of Pal-GC12: Pal-GLLSGINAEWPC, molecular weight: 1497.86, and its structural formula is as follows:
[0062]
[0063] The preparation of Pal-GC12, the specific steps are as follows:
[0064] 3.1. Weigh 2.5 g (1 mmol) of H-Cys(Trt)-2-Chlorotrityl Resin resin (Sub=0.40 mmol / g) into the reaction column, wash with DMF 3 times, and swell with DMF for 30 minutes. Weigh 2.5 g (6 mmol) of Fmoc-Pro-OH and 0.9 g (6.6 mmol) of HOBt, dissolve them in DMF, add 1.2 g of DIC (9 mmol) in an ice-water bath at 0°C, activate for 5 minutes, add to the reaction column, and react for 2 hours. The Fmoc protecting group was then removed with DBLK. Repeat the above operation, according to the sequence coupling Fmoc-Trp(Boc)-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Ala-OH, Fmoc-Asn(Trt)-OH, Fmoc-Ile-OH, Fmoc-Gly -OH, Fmoc-Ser(tBu)-OH, Fmoc-Leu-OH, Fmoc-Leu-OH, Fmoc-Gly-OH, Palmitoyl chloride, washed with DMF, DCM, methanol in turn after the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com