Biological fermentation preparation method of semaglutide core peptide chain
A bio-fermentation and peptide chain technology, which is applied in the field of preparation of semaglutide core peptide chain, can solve the problems of high cost of preparation route and high price of endonuclease market, and achieve the effect of convenient purification, less impurities and lower difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The DNA sequence synthesis of embodiment 1 fusion protein A1 and the construction of plasmid
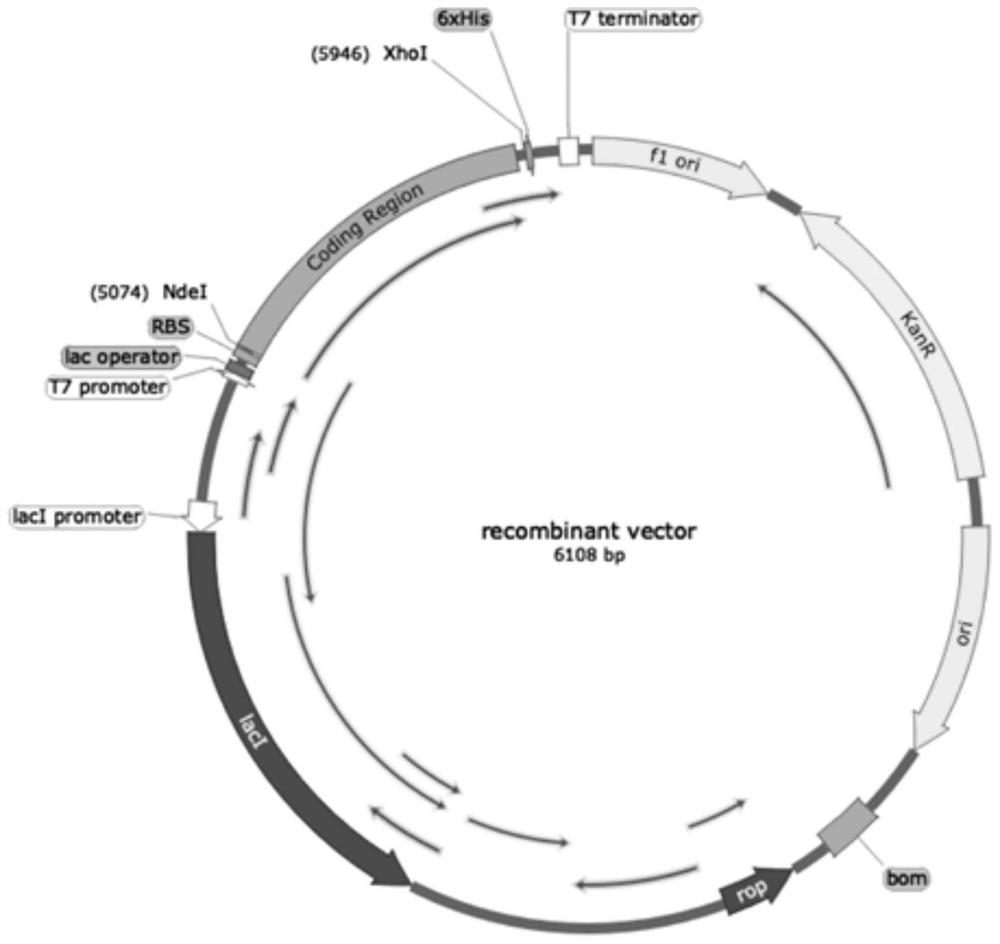
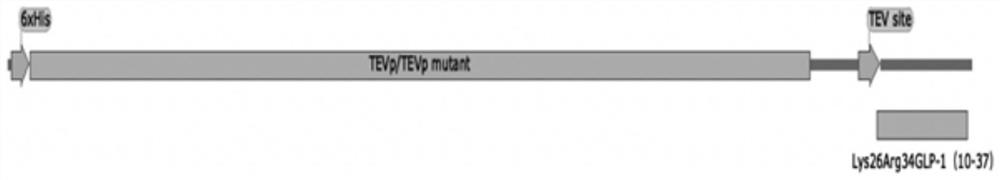
[0028] Total synthesis of TEVp 238△mutant-Lys by Shanghai Jierui Biotechnology Co., Ltd. 26 Arg 34 GLP-1(10-37) DNA sequence, add His tag to the N-terminus of the sequence, add TGA to the C-terminus, design primer F: CATATG CATCATCATCATCATCATGGCGAATCTCTGT,R: CTCGAG TTAGCCCCACGACCACGCA (as shown in SEQ ID NO: 6-7), and link the target sequence to the vector, the fusion protein sequence is shown in SEQ ID NO: 1, and its structure is shown in figure 2 , constructed to express His tag-TEVp238△mutant-TEV protease site-Lys 26 Arg 34 The recombinant fusion expression vector of GLP-1(10-37), such as figure 1 shown. Among them, TEVp 238△mutant is one of the TEVp mutants.
Embodiment 2
[0029] Embodiment 2 Expression Engineering Bacteria Preparation and Positive Verification
[0030] After the recombinant expression vector in Example 1 was validated by gel running, it was transformed into E.coli BL21 competent medium, positive colonies were screened out by Km antibiotics, full and positive colonies were selected, and DNA sequencing verification was carried out after expansion, and after confirmation, proceed Glycerol bacteria preservation.
Embodiment 3
[0031] Embodiment 3 Expression of fusion protein A1
[0032] The preserved recombinant strains were fermented in a 15L fermenter and cultivated to OD at 37°C 600 At 20 o'clock, IPTG was added for induction, the amount of IPTG added was 0.2mM, and the strain OD was detected at 0h after induction 600 , induced to OD 600 If the increase is not obvious, put it into the tank, centrifuge at 6000rpm for 10min, and collect the bacteria.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com