Cypemycin precursor peptide mutant and application thereof and prepared cypemycin analogues
A technology of sepramycin and precursor peptide, applied in the field of biotechnology engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Construction of recombinant plasmids containing mutant precursor peptides
[0063] Firstly, use the PCR method, using forward CypA-F and reverse CypA-R as primers, Streptomyces sp.OH4156 (references: Claesen J, Bibb M. Genome mining and genetic analysis of cypemycin biosynthesis reveal an unusual class of posttranslationally modified peptides. [J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(37): 16297-16302.) total DNA is template amplification target fragment cypA (nucleic acid sequence such as SEQ ID NO.27 in the sequence listing shown); the PCR reaction conditions were: pre-denaturation at 95°C for 1 min; denaturation at 95°C for 15 s; annealing at 58°C for 15 s; extension at 72°C for 20 s; extension at 72°C for 7 min; cycle 30 times.
[0064]Two strategies are mainly used to design point mutation primers: if the target amino acid to be mutated is located at an important amino acid at the C-terminal of the cor...
Embodiment 2
[0069] Example 2 Conjugative Transfer Obtaining a Bacterial Strain Containing a Mutant Propeptide
[0070] The vector pIJ10257 was digested simultaneously with restriction endonucleases HindIII and NdeI (see: Claesen J, Bibb M. Genome mining and genetic analysis of cypemycin biosynthesis reveal anunusual class of posttranslationally modified peptides. [J]. Proceedings of the National Academy of Sciences of the United States of America, 2010,107(37):16297-16302.) and DNA amplified fragment cypA, construct plasmid pHB-CypA-wt, clone plasmid into competent cell E.coli ET12567 (commercially available obtained) and combined with the recipient bacterial cell S.coelicolor M1414(ΔcypA) (construction method reference: Claesen J, Bibb M. Genome mining and genetic analysis of cypemycin biosynthesis reveal an unusual class of posttranslationally modified peptides.[J].Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(37): 16297-16302.) The wild-type...
Embodiment 3
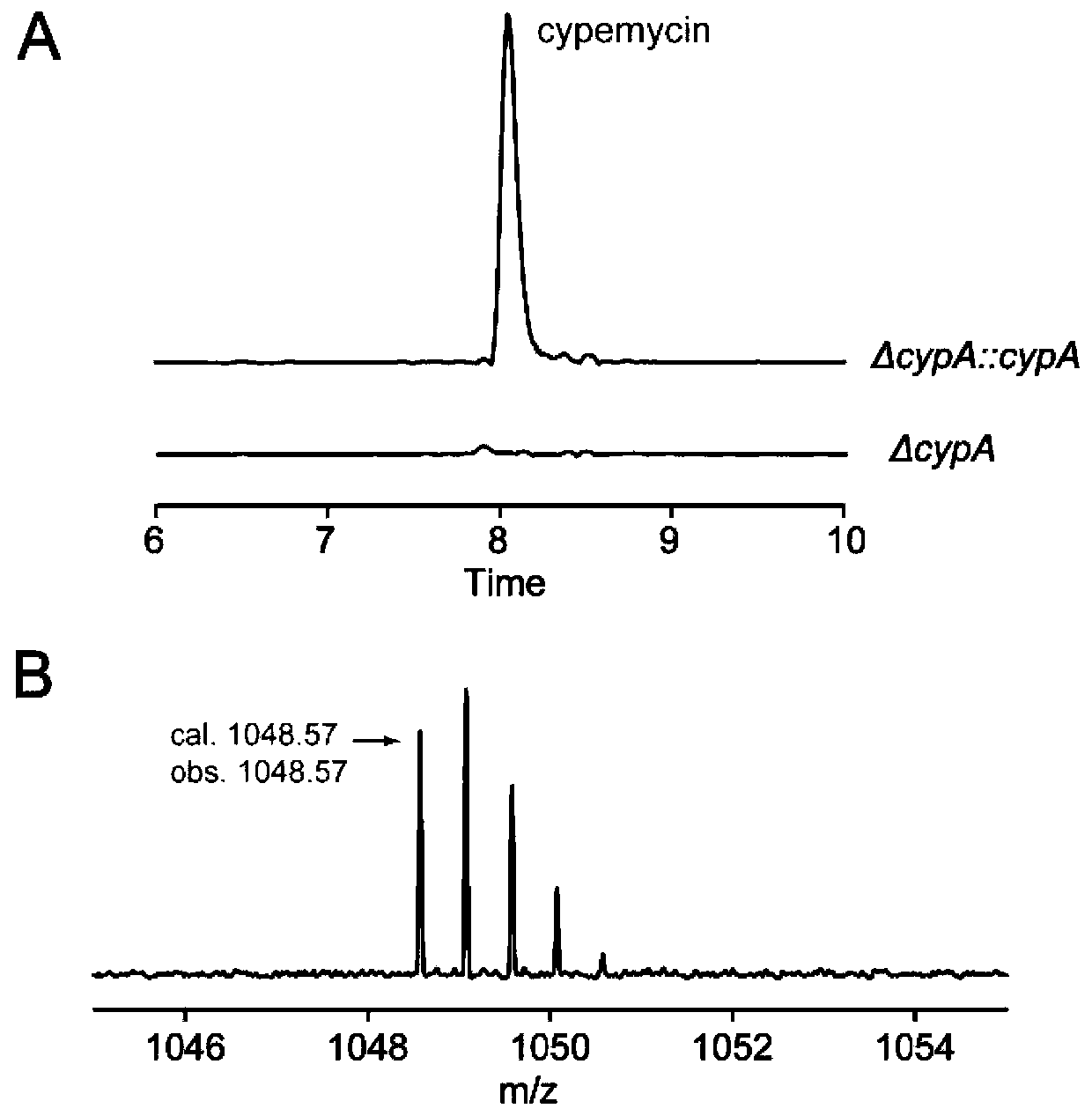
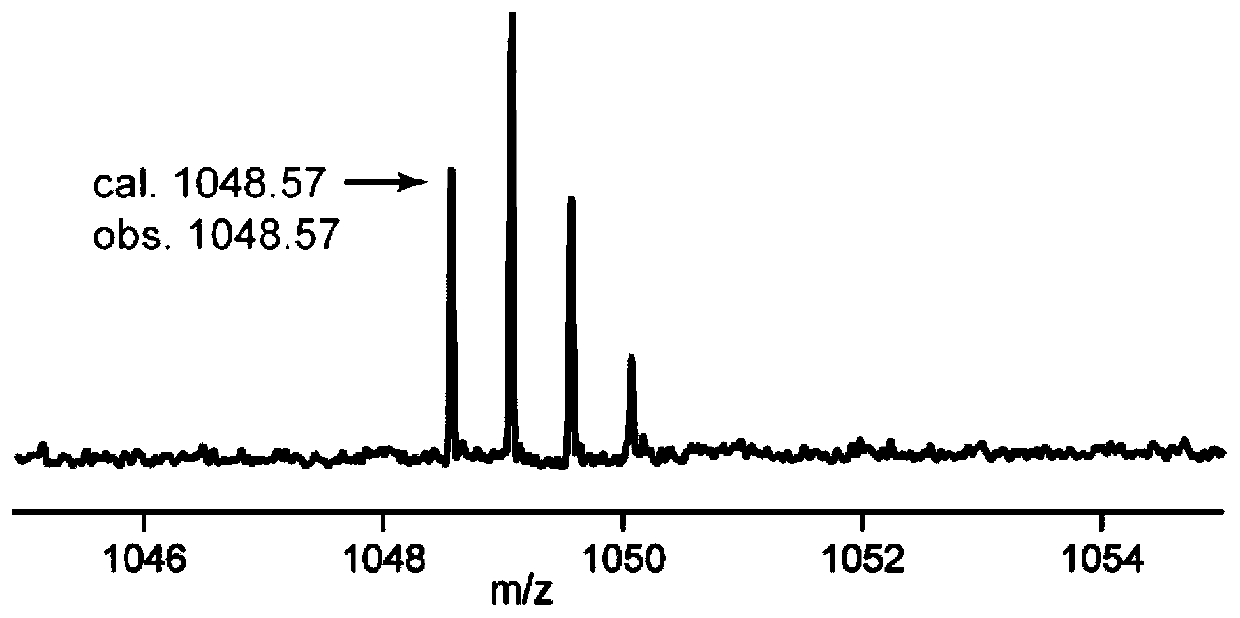
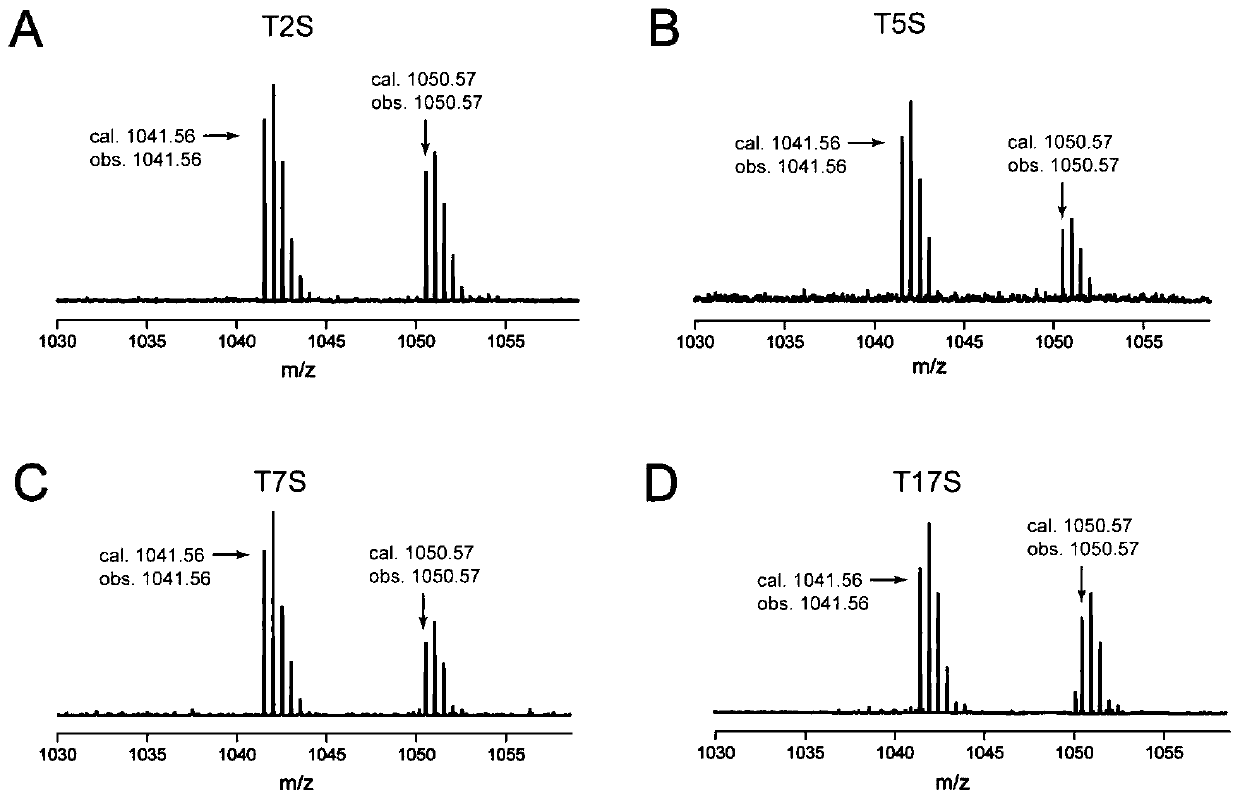
[0072] Example 3 Obtaining Sepromycin and Sepromycin Structural Analogues by Fermentation
[0073] (1) Optimization of wild-type and mutant fermentation conditions: analysis of liquid phase mass spectrometry (LC-MS) experiment results found that the yield of septomycin was very low, which was not conducive to the analysis of septomycin and its structural analogues. To improve the yield of septomycin, it is urgent to further optimize the fermentation conditions of the replenished wild-type strain S.coelicolor M1422, set the volume of fermentation broth (100mL), the volume of extraction solvent (100mL), and the volume of solvent for extracting mycelium (100mL) must, on the basis of the septomycin fermentation medium MarM (for details, see: Komiyama, K., Otoguro, K., Segawa, T., Shiomi, K., Yang, H., Takahashi, Y., Hayashi, M., Oxani, T., and Omura, S. (1993) A new antibiotic, cypemycin. Taxonomy, fermentation, isolation and biological characteristics. J. Antibiot. 46, 1666-1671)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com